Mitral Valve Disease and
the Cavalier King
Charles Spaniel
-
 IN SHORT
IN SHORT - IN DEPTH
- WHAT IT IS
- SYMPTOMS
- DIAGNOSIS
- DNA TESTING
- INFLAMMATION
- STAGES of MVD
- PROGRESSION & PROGNOSIS
- TREATMENT OTHER THAN MEDICATION
- -- dietary treatment
- -- heart supplements
- MEDICATIONS
- -- Stage B1
- -- Stage B2
- -- Stage C
- -- Stage D
- SURGERY
- -- cardiopulmonary bypass (CPB) surgeries
- -- transcatheter edge-to-edge device (TEER)
- -- transapical beating heart mitral valve replacement
- -- other minimally invasive surgeries
- ANESTHESIA
- TRICUSPID VALVE DISEASE
- BREEDERS' RESPONSIBILITIES
- WHAT YOU CAN DO
- -- annual heart checks
- -- when to get that first chest x-ray
- -- count the breaths per minute
- -- avoid vaccines
- RESEARCH NEWS
- RELATED LINKS
- VETERINARY RESOURCES
- PAGE 2 of MVD
- PAGE 3 of MVD
IN SHORT:
 Heart
mitral valve disease (MVD) is the leading cause of death of cavalier King
Charles spaniels throughout the world. MVD is a polygenetic disease which
statistics have shown may afflict over half of all cavaliers by age 5 years
and nearly all cavaliers by age 10 years, should they survive that long. MVD
has been found to be 20 times more prevalent in CKCSs than in the average
dog breed. It is estimated to affect 10% of the entire dog population,
but at a much older age of onset than for CKCSs. In the United States, out
of 300,000 dogs, 5% died of MVD while 50% of the cavaliers died of MVD.
More ...
Heart
mitral valve disease (MVD) is the leading cause of death of cavalier King
Charles spaniels throughout the world. MVD is a polygenetic disease which
statistics have shown may afflict over half of all cavaliers by age 5 years
and nearly all cavaliers by age 10 years, should they survive that long. MVD
has been found to be 20 times more prevalent in CKCSs than in the average
dog breed. It is estimated to affect 10% of the entire dog population,
but at a much older age of onset than for CKCSs. In the United States, out
of 300,000 dogs, 5% died of MVD while 50% of the cavaliers died of MVD.
More ...
What It Is
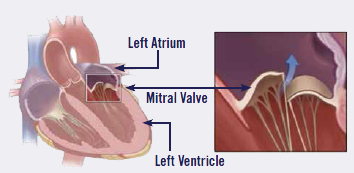
 MVD is a degeneration of the heart's mitral valve, one of four sets of
valves in a dog's heart. A dog's heart valves' leaflets must open and
close tens of thousands of times a day to maintain uni-directional blood
flow through the heart. When the valves open, they direct blood flow
forward to where it is supposed to go, and when they close, they prevent
blood from going backward to where it is not supposed to go. The mitral
valve is located between the left atrium and ventricle.
MVD is a degeneration of the heart's mitral valve, one of four sets of
valves in a dog's heart. A dog's heart valves' leaflets must open and
close tens of thousands of times a day to maintain uni-directional blood
flow through the heart. When the valves open, they direct blood flow
forward to where it is supposed to go, and when they close, they prevent
blood from going backward to where it is not supposed to go. The mitral
valve is located between the left atrium and ventricle.
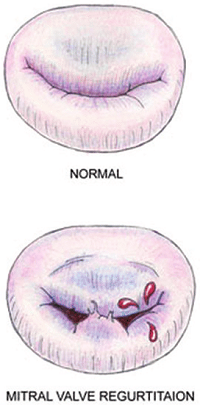
As the mitral valve degenerates, the valve no longer fully closes after each pumping action, allowing some blood to flow backwards through them from the ventricle back into the atrium. As the condition worsens, more and more blood is able to backflow through the valve as the leaflets of the valve begin to flail. In the final stages, the valve's struts (chordae tendineae) sometimes break, causing the valve to collapse completely. In most dogs affected with MVD, the disease seldom progresses to heart failure. The estimates have varied from 20% to 30% of all dogs diagnosed with MVD eventually going into heart failure. However, MVD usually results in heart failure in the CKCS.
Heart failure (HF) is a condition where the heart is still working, but it can't pump enough blood to fully meet the dog's body's needs. HF is determined by its symptoms, which include high rates of breathing (respiratory rates), exercise intolerance, shortness of breath (dyspnea), increase in respiratory effort, and/or fainting. This form of heart faiure also is referred to as "forward heart failure". The term "congestive heart failure " (CHF) refers to the heart's dysfunction causing fluid buildups in the lungs (pulmonary edema) or elsewhere (effusions). Congestive heart failure (CHF) is the next step in the progression of MVD, following heart failure (HF).
About 10% of all dogs suffer from some form of heart disease. Mitral valve disease is the most common heart disorder in older dogs of all breeds. However, in the cavalier King Charles spaniel, the prevalence of MVD is about 20 times that of other breeds. Also in cavaliers, the onset of the disease typically is much earlier in the life of the dog. It has been reported that, once diagnosed, mitral valve disease is much more rapid in cavaliers than in other breeds, possibly reaching a life-threatening stage within as little as 1 to 3 years, rather than the average 3 to 5 years. To a lesser extent, cavaliers also suffer from deterioration of their tricuspid valves. More...
Diagnosis
 All cavaliers should be screened for
the sounds of tubulent blood flow, called heart murmurs, once a year beginning
at age 1 year. Once MVD is detected, its progression can be monitored with stethoscopic examinations (auscultations), x-rays, echocardiograms, and
color Doppler echocardiograms. If a heart murmur is detected, it should be
confirmed in 3 to 6 months. If it still is detected, the dog is considered
probable for MVD. More...
All cavaliers should be screened for
the sounds of tubulent blood flow, called heart murmurs, once a year beginning
at age 1 year. Once MVD is detected, its progression can be monitored with stethoscopic examinations (auscultations), x-rays, echocardiograms, and
color Doppler echocardiograms. If a heart murmur is detected, it should be
confirmed in 3 to 6 months. If it still is detected, the dog is considered
probable for MVD. More...
Symptoms & Treatment
The progression of mitral valve disease can be rapid or slow. In most cavaliers, the disease shows a gradual progression in the loudness of the murmur and to more serious symptoms, in as little as 2 years after first detecting the murmur. Drugs may help to minimize the symptoms, but eventually the drugs may be unable to control them. The drugs prescribed for cavaliers with MVD can sometimes have severe adverse side effects, and blood chemistry should be done routinely to monitor their effects upon the kidneys, liver, and other internal organs. Severe symptoms of MVD in some cavaliers will appear more quickly, although previously having been stable. The ultimate consequence of the disease is heart failure. More...
Breeders' Responsibilities
Early-onset mitral valve disease has been found to be "highly heritable" in the cavalier King Charles spaniel breed, and "selection against the disease should be successful.", according to an April 2011 research report.
Due to the pervasiveness of MVD in the breed worldwide, cavalier King Charles spaniels under the age of five years should not be bred (with one limited exception -- see MVD Breeding Protocol). Also, no cavalier should be bred after age five years if it developed an MVD murmur before the age of five years. Any littermates of breeding stock having early-onset MVD (mitral valve murmurs before age 5 years) should be taken into very serious consideration. All CKCS breeding stock should be examined by board certified veterinary cardiologists at least annually and cleared by the veterinary specialists for MVD, the closer the examination to the breeding the better. It is recommended that all cavaliers, breeding stock or not, be examined annually by board certified veterinary cardiologists after age one year. See the current list of health clinics for upcoming cardiologist examinations.
RETURN TO TOP
IN DEPTH:
 Degenerative mitral valve disease (MVD)* is the leading cause of death of
cavaliers. It is a highly-heritable, polygenetic acquired heart disease which, statistics
show, afflicts over half of all cavalier King Charles spaniels by age 5
years (by stethoscopic examination) and
greater than 90% by age 10+ years, should they survive that
long. It is estimated to affect 10% of the entire dog population, but at a
much older age of onset than for CKCSs.
Degenerative mitral valve disease (MVD)* is the leading cause of death of
cavaliers. It is a highly-heritable, polygenetic acquired heart disease which, statistics
show, afflicts over half of all cavalier King Charles spaniels by age 5
years (by stethoscopic examination) and
greater than 90% by age 10+ years, should they survive that
long. It is estimated to affect 10% of the entire dog population, but at a
much older age of onset than for CKCSs.
* MVD is also called cardiac valve disease (CVD) and medically known as myxomatous mitral valve disease (MMVD) chronic degenerative valvular disease, chronic valvular disease, chronic mitral valve insufficiency, myxomatous atrioventricular degeneration, endocardiosis, atrioventricular valve endocardiosis, chronic valvular fibrosis, acquired mitral regurgitation or insufficiency, and mitral valve defect.
Veterinary cardiologists began compiling statistics on cavaliers with MVD murmurs in the United Kingdom in 1990. In a 1993 study of 394 cavaliers in the USA, 9% of puppies under age 12 months had MVD murmurs. 100% of CKCSs at age 10 years or older had MVD murmurs. 56% of cavaliers under age 5 years had MVD murmurs.
Since then, cardiologists have examined the hearts of many thousands of cavalier King Charles spaniels at health clinics held by CKCS breed clubs in the UK, Canada, the USA, and elsewhere. From the data they have compiled, they have found that the percentage of CKCSs which develop MVD murmurs increases at a rate of about 10% per year. So, roughly 10% of cavaliers by age one year have MVD murmurs, and 20% aged between one and two years have murmurs, and so on for each age level. Specifically, the statistics show that more than half of all cavaliers aged five years have murmurs, and it is the very rare cavalier at age ten years which does not have, at the very least, a low grade MVD murmur.
A pair of September 2005 studies [1] [2] of Swedish cavaliers showed that 23% were dead by eight years, and 48% were dead by ten years. Those researchers stated:
"Heart disease in the Cavalier King Charles spaniel accounts for over 50% of deaths in that breed (in dogs under 10 years of age) and for over one-quarter of the heart deaths in the insured population [of all breeds]. Although heart disease in Cavalier King Charles spaniels is well recognized, these statistics give further insight into the impact of this cause of death in this breed."
In the United States, out of 300,000 dogs, 5% died from MVD while 50% of the cavaliers died from MVD. In a 2006 study comparing the severity of MVD in cavaliers with six other breeds (Bichon, Dachshund, Lhassa Apso, poodle, Shi Tzu, and Yorkshire terrier), the researchers reported that in those other breeds, the age of onset of MVD is much later, and MVD is a well-tolerated disease with a long and slow progression, with most of the dogs not reaching heart failure. In a July 2017 article, the authors described MVD as a "relatively benign condition" in most breeds of dogs, with the exception of cavaliers.
RETURN TO TOP
What It Is
- Mitral valve leaflets
- Chordae tendineae
- Mitral valve shape (morphology)
- Mitral valve prolapse
- Mitral annular disjunction
- Compensatory mechanisms
- Renin-angiotensin-aldosterone system (RAAS)
- Progression of MVD
- Mitral valve dysplasia
- Other types of heart disorders
 Mitral valve disease is a uniquely serious, life-shortening problem for
cavalier King Charles spaniels and is their leading cause of death. About
10% of all dogs suffer from some form of heart disease. MVD is
the most common heart disorder in older dogs of all breeds. Several smaller breeds of
dogs typically are predisposed to suffer from MVD. However, in most all
breeds, MVD does not result in heart failure (HF), causing death, because MVD
does not develop early in a dog's life, and does not progress rapidly.
See this
January 2008 article, in which, of 302 MVD-affected dogs of various
breeds with mild heart enlargement, over 60% were alive 70 months after
initial diagnosis, and over 70% never reached the stage of heart failure.
Mitral valve disease is a uniquely serious, life-shortening problem for
cavalier King Charles spaniels and is their leading cause of death. About
10% of all dogs suffer from some form of heart disease. MVD is
the most common heart disorder in older dogs of all breeds. Several smaller breeds of
dogs typically are predisposed to suffer from MVD. However, in most all
breeds, MVD does not result in heart failure (HF), causing death, because MVD
does not develop early in a dog's life, and does not progress rapidly.
See this
January 2008 article, in which, of 302 MVD-affected dogs of various
breeds with mild heart enlargement, over 60% were alive 70 months after
initial diagnosis, and over 70% never reached the stage of heart failure.
In the cavalier King Charles spaniel, statistics have shown that the prevalence of MVD is about 20 times that of other breeds of dog. Also in cavaliers, the onset of the disease typically is much earlier in the life of the dog, with over half of all CKCSs having developing MVD by their fifth birthday (by stethoscopic examination), as noted above. For nearly all other breeds, MVD is an old-age disease, and the age of onset is between 10 and 15 years of age. Prof. Melanie Hezzell stated in her June 2025 article, "The lifetime prevalence of MMVD in CKCS approaches 100 per cent, whereas the prevalence of the disease in non-CKCS breeds is around 14 per cent."
In most dogs affected with MVD, the disease seldom progresses to heart failure. The estimates have varied from 20% to 30% of all dogs diagnosed with MVD eventually going from heart enlargement into heart failure. However, MVD usually results in heart failure in the CKCS.
It has been reported that, once diagnosed, MVD is much more rapid in cavaliers than in other breeds, possibly reaching a life-threatening stage within as little as 1 to 3 years, rather than the average 3 to 5 years. Studies of cavaliers have concluded that it has an hereditary basis and is "polygenetic", meaning that more than one gene can be the cause.
Some research has indicated that MVD in the CKCS may be attributed to a chronic state of inflammation, as evidenced by measurements of immunoglobulin antibodies and glycoprotein and complement proteins particularly associated with immune responses to inflammation. See this 2014 Italian study. In a 2006 USA study, researchers found that, compared with controls, dogs with chronic valvular disease had higher plasma concentration of C-reactive protein (CRP). In veterinary medicine, CRP concentration has been shown to increase in inflammatory states, such as pancreatitis.
Other research by Dr. Brendan Corcoran indicates that the damaging of the CKCS mitral valves is due to a life-long traumatic condition combined with the dog's inability to appropriately repair that damage. He has coined the term, "dyscollagenesis" (as opposed to fibrosis) meaning a chronic reduction in collagen production and a disorganization and failure of maturation. See his 2010 report. He also has found that the progression of the degeneration of the mitral valve may be controlled by TGFB (transforming growth factor beta) transforming the nature of the valve's cells.
RETURN TO TOP
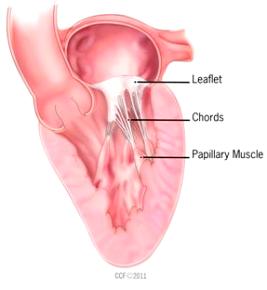
Mitral valve leaflets
MVD is a degeneration and fibrosis of the heart's mitral valve, one of
four sets of valves in a canine's (and a human's) heart. It is the valve
which is designed to prevent the backflow of blood from the left ventricle
into 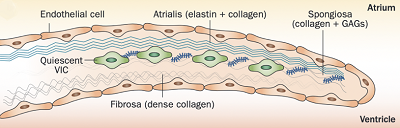 the left atrium (called mitral regurgitation -- MR).
The normal mitral valve has a saddle shape. It consists of a set of double flaps, called
"leaflets" or
"cusps", that open and close like a set of one-way doors at appropriate
times during each heart beat, together with a mitral ring which surrounds
the leaflets, the chordae tendineae,
and the papillary muscles.
the left atrium (called mitral regurgitation -- MR).
The normal mitral valve has a saddle shape. It consists of a set of double flaps, called
"leaflets" or
"cusps", that open and close like a set of one-way doors at appropriate
times during each heart beat, together with a mitral ring which surrounds
the leaflets, the chordae tendineae,
and the papillary muscles.
Normal mitral valve leaflets (see diagram of a leaflet cross-section, above at right) consist of four layers of tissue (atrialis, fibrosa, spongiosa, and ventricularis), most of which are comprised of collagen and elastin fibers, and are very thin and nearly transparent. The two leaflets are the "anterior" (front) leaflet and the "posterior" (rear) leaflet. They are connected by the chordae tendineae to the papillary muscles of the left ventricle.
Blood flows through the pulmonary veins from the lungs into the left
atrium, one of the chambers of the heart.
 The mitral valve is located
between the left atrium and the left ventricle, another chamber in the
heart. The valve's action is governed by the movement of blood as it is
pumped from the atrium and into the ventricle. The two leaflets of the mitral
valve are controlled by the tendons -- the chordae tendineae -- which serve as thin "struts" shaped
much like the chords of a parachute. Normal healthy chordae are smooth and
symmetrical.
The mitral valve is located
between the left atrium and the left ventricle, another chamber in the
heart. The valve's action is governed by the movement of blood as it is
pumped from the atrium and into the ventricle. The two leaflets of the mitral
valve are controlled by the tendons -- the chordae tendineae -- which serve as thin "struts" shaped
much like the chords of a parachute. Normal healthy chordae are smooth and
symmetrical.
As the diseased mitral valve
degenerates, myxomatous transformation -- the development of excess gel-like connective
tissue between the cells of the leaflets, the extracellular matrix -- causes the valve to lose its flexibility, its
leaflets thickening and shortening, its fibers stiffening, and its
chordae tendineae elongating. The leaflets develop nodules which appear
greyish white, smooth, and glistening, as
 if filled with fluid. As they
increase in number and size, their effect upon the chordae tendineae and
the function of the valve worsens. (See image at right, from this
March 2012 article.) These nodules, called lesions, are
graded according to their severity from Type I to Type IV, called the
"Whitney grades". See this
August 1974 article for their details. See, also, this
January 2010 article for a more detailed description of the changes
in the valve leaflets as MVD progresses.
if filled with fluid. As they
increase in number and size, their effect upon the chordae tendineae and
the function of the valve worsens. (See image at right, from this
March 2012 article.) These nodules, called lesions, are
graded according to their severity from Type I to Type IV, called the
"Whitney grades". See this
August 1974 article for their details. See, also, this
January 2010 article for a more detailed description of the changes
in the valve leaflets as MVD progresses.
Eventually, the leaflets no longer fully close after each pumping action, allowing blood to jet backwards through them from the ventricle back into the atrium. This is the mitral regurgition (MR). (In the diagram at the upper left, a healthy mitral valve at the top is compared with a damaged valve below.)
In a March 2012 article reviewing the history of research into MVD, Drs. Michele Borgarelli and James W. Buchanan wrote:
"The mitral valve is probably the most abused and stressed tissue in the body because it is intermittently bent, slammed, tensed, shear stressed and stretched, 50-200 times a minute, 24 hours a day, 365 days a year for 10-15 years."
RETURN TO TOP
Chordae tendineae
 The two leaflets of the mitral valve, the anterior
(front) leaflet and the
posterior (rear) leaflet, are connected by tendons, called chordae tendineae
(CT or chords),
to the papillary muscles of the left ventricle. An average of 24 chordae are
attached to the anterior leaflet and 18 to the posterior leaflet. There
are three different types of CTs:
The two leaflets of the mitral valve, the anterior
(front) leaflet and the
posterior (rear) leaflet, are connected by tendons, called chordae tendineae
(CT or chords),
to the papillary muscles of the left ventricle. An average of 24 chordae are
attached to the anterior leaflet and 18 to the posterior leaflet. There
are three different types of CTs:
• first-order CTs, connecting the leaflets to the papillary muscles;
• second-order CTs, which are located between the surface of the leaflets and the papillary muscles; and
• third-order CTs, which extend between the posterior leaflet and the wall of the left ventricle.
They also are classified as major and minor, based upon their relative sizes and strengths. The CTs transmit the papillary muscles' contractions and relaxations to the leaflets. CTs consist of collagen and elastic fibers.
Chordae may fail due to elongation, rupture, thickening, retraction, or calcification. Mitral valve prolapse (MVP) may cause the valve's chordae tendineae to stretch. In the final stages, the valve's chordae tendineae sometimes rupture, and if they are major chords, causing the valve to collapse completely. Rarely, a cavalier's mitral valve's major chordae tendineae may suddenly rupture earlier in the progression of the disease, before any enlargement of the heart takes place.
In this February 2025 article, in which 42 canine mitral valves were examined, the investigators found that CTs degenerate along with the rest of the valve in dogs affected with MVD. As the CTs degenerate, their fibers become uneven and their elastic properties and the value of their tensile strength decrease, causing the CTs to more lkely rupture. Read more about rupture of the chordae tendineae below at this link.
RETURN TO TOP
Mitral valve shape (morphology)
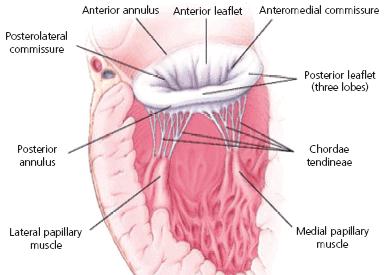
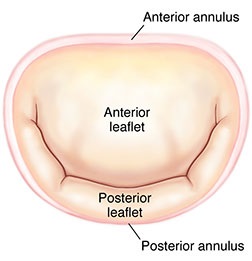 The
mitral valve's two leaflets are surrounded by a ring called the
annulus (MVA). (See image at right.) The dog's MVA
contracts (systolic phase -- when the heart is pumping
blood the arteries) and expands (diastoic phase -- when
the heart refills with blood). This is called the cardiac cycle.
In most healthy dogs without MVD, their MVA is saddle-shaped or
elliptical but during the diastolic phase, the annulus becomes rounder.
The
mitral valve's two leaflets are surrounded by a ring called the
annulus (MVA). (See image at right.) The dog's MVA
contracts (systolic phase -- when the heart is pumping
blood the arteries) and expands (diastoic phase -- when
the heart refills with blood). This is called the cardiac cycle.
In most healthy dogs without MVD, their MVA is saddle-shaped or
elliptical but during the diastolic phase, the annulus becomes rounder.
3-D echos* have enabled cardiologists to more clearly observe the functioning of the mitral valve, including "tethering" of the valve's leaflets, and the dimensions of the "tenting" area created during the tethering. In some instances, 3-D echo measurements of cavaliers' mitral valves have shown that CKCSs' valves structural features differ from those of other breeds, which may explain why the onset of MVD in cavaliers is earlier and progresses more rapidly than in the average canine. In a September 2016 abstract, an international panel of cardiologists used 3-D echocardiography to compare the mitral valves of 22 cavalier King Charles spaniels with 41 other dogs of 18 different breeds. They measured the dimensions of the mitral valve's annulus (see diagram at right), tenting (see diagram at left below), leaftet areas, and several other categories.
* 3-D echos are technically referred to as real-time transthoracic three-dimensional echocardiography analysis (RT3DE).
They found that cavaliers had significantly smaller annulus diameter,
annulus height, tenting height, tenting area, normalized tenting volume,
posterior leaflet length, normalized posterior leaflet area, and a
greater annulus sphericity index. They concluded that the mitral valve
of healthy CKCSs was more circular and had less tenting, compared to
other
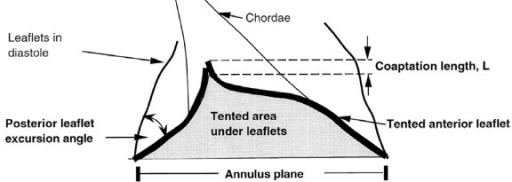 breeds.
breeds.
The echocardiograph examination shows the dimensions of the heart chambers, wall thickness and movement, valve movement and lesions, fractional shortening, among other characteristics. The echo screen shows the amount of wall contraction, which enables the operator to determine contractility, preload*, and afterload*. These factors are used to calculate "fractional shortening" (FS%) which is used as an indication of ventricular performance and of myocardial contractility.
* Preload is the blood filling the left ventricle, thereby stretching the heart muscle cells before contraction. Afterload is the blood contained in the left ventricle against which the heart contracts to eject that blood into the arteries.
See, also, this March 2017 article, in which the same investigators used 3-D echocardiography analysis on 113 dogs, including 13 cavaliers affected in varying stages of MVD. The 3-D echos enabled the investigators to compare the morphology of the mitral valves (MVs) of healthy dogs (none were CKCSs) and MVD-affected dogs. They report that the study demonstrated that the MVs of MVD-affected dogs differed from those of healthy dogs in several morphological aspects. In particular, the affected dogs had an increased sphericity and a decreased saddle shape of the MV annulus, as well as a decreased tenting height, area and volume. See Figure 1. The study also reportedly demonstrated significant differences in multiple 3-D echo MV measurements between dogs in varying stages (B1, B2, C) of MVD.
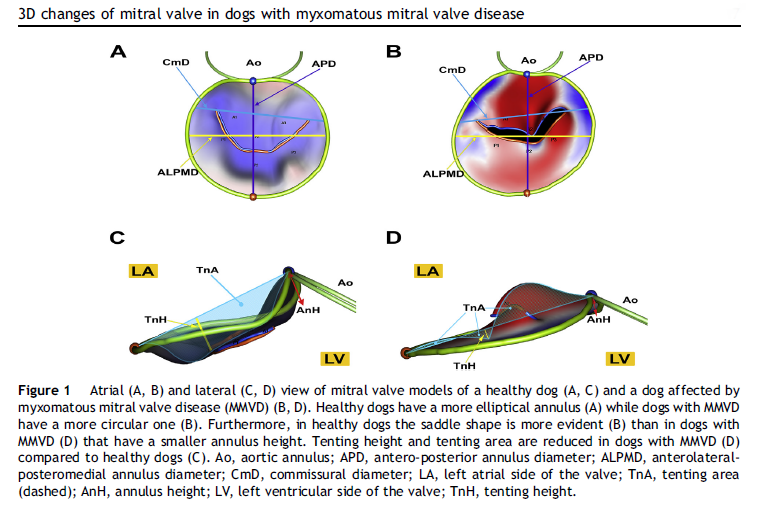
In an April 2021 article, Japanese cardiology researchers used three-dimensional transesophageal echocardiography (TEE) to examine 31 MVD-affected dogs, including 9 in Stage B2, 15 in Stage C, and 7 in Stage D. The TEE was performed while the dogs were under anesthesia prior to mitral valve repair surgeries. They found that the annulus height to commissural width ratio of Stage D dogs had significantly lower values than Stage B2 dogs, and that the aortic-mitral angle of Stages C and D dogs were significantly flatter than those in Stage B2. They concluded that the saddle shape of the mitral annulus and aortic-mitral angle were flatter in Stage D than those in the other two stages.
In an August 2021 study, Japanese cardiologists studied the shapes of the MVA in 59 healthy dogs and 371 MVD-affected dogs about to undergo mitral valve surgery. They reported finding that two-dimensional echocardiography revealed that the MVAs of the healthy dogs were elliptical and the annuluses of the MVD-affected dogs were larger than those of healthy dogs, and the annuluses remained rounder during the full cardiac cycle.
In a September 2022 abstract presented at the 32d European College of Veterinary Internal Medicine - Companion Animals (ECVIM-CA) Congress, a team of cardiologists at the Virginia-Maryland College of Veterinary Medicine (G. Menciotti, M. Borgarelli, A. Franchini, S.M. Lahmers, H.W. Jeong) used transthoracic three-dimensional echocardiography (3DTTE) to examine the shapes of mitral valves in 80 cavalier King Charles spaniels, 41 of which had no murmur, and the other 39 had grade 1 or grade 2 murmurs. They found that the shapes of the mitral valves of the MVD-affected cavaliers were "significantly different" from those with no murmurs. The mitral valves of the MVD-affected CKCKs had wider diameters, circumferences, and areas, and both of their mitral valve leaflets had larger areas and lengths. Also, the angle between both anterior and posterior leaflets and the annulus (ring around the valve) was flatter in the MVD-affected dogs. They concluded:
"These findings indicate that CKCS with only mild MR [mitral regurgitation] have MV [mitral valve] morphological differences compared to CKCS without MR. This further supports previous research suggesting a role of MV morphology in the pathophysiology of MMVD, and further investigation of a causative link is warranted."
RETURN TO TOP
Mitral valve prolapse
 Mitral valve prolapse (MVP)* describes the
displacement of the mitral leaflets from their normal position in
relation to the mitral anulus.
The normal mitral valve is saddle-shaped, and when the valve is closed,
the two leaflets meet tightly together, as shown in the "normal" image
at right.
Mitral valve prolapse (MVP)* describes the
displacement of the mitral leaflets from their normal position in
relation to the mitral anulus.
The normal mitral valve is saddle-shaped, and when the valve is closed,
the two leaflets meet tightly together, as shown in the "normal" image
at right.
As the MVD condition worsens, advanced lesions cause the leaflets to fold, invert, and stretch toward the left atrium. This eventually causes MVP. In some cavaliers, MVP may be the first sign of possible MVD, even before a mild mitral valve murmur is detected. On ultrasound (echocardiograph) examination, MVP appears as if the valve leaflets overlap backwards into the atrium.
*Mitral valve prolapse (MVP) is defined as the protrusion of one or both valve leaflets to the atrial side of the plane of the mitral annulus during systole (1999 study). The degree of MVP correlates with the severity of mitral valve regurgitation. Peddle GD, Buchanan JW. Acquired atrial septal defects secondary to rupture of the atrial septum in dogs with degenerative mitral valve disease. J. Vet. Cardiol. 2010;doi: 10.1016/j.jvc.2010.03.002
In a 1999 study, echocardiographic screening of 75 cavaliers revealed that 82% (54/66) of those dogs aged one to three years had MVP, and 97% (84/87) of the dogs over three years had various degrees of mitral valve prolapse. In a September 2021 Italian study, 51 of 52 cavaliers had MVP, including 25 CKCS which had no detectable mitral valve murmurs.
MVP may cause the valve's chordae tendineae to stretch. In the final stages, the valve's chordae tendineae sometimes rupture, and if they are major chords, causing the valve to collapse completely. Rarely, a cavalier's mitral valve's major chordae tendineae may suddenly rupture earlier in the progression of the disease, before any enlargement of the heart takes place.
RETURN TO TOP
Mitral annular disjunction
A further consequence of progression of MVD beyond changes in the morphology of the mitral valve leaflets and mitral valve prolapse is mitral annular disjunction (MAD). This describes a separation between the posterior (rear) mitral valve leaflet and the atrial wall. It is known to exist in dogs only in cases of advanced MVD, and even then it is quite rare, with a prevalence of only 2.5% among dogs diagnosed with MVD and MVP in a June 2025 article.
RETURN TO TOP
Compensatory mechanisms
The initial effect of MVD upon the affected dog's body is a very mild reduction in the heart's output of blood (cariac output) along with reductions in blood pressure and dissolved oxygen (oxygen tension) in the blood stream. These signals prompt other regions of the dog's body to initiate compensatory mechanisms aimed at maintaining normal cardiac output, blood pressure, and oxygen tension levels. These mechanisms include:
(a) activating the sympathetic nervous system to increase the heart rate to normalize cardiac output and constrict the blood vessels to normalize blood pressure;
(b) activating the kidneys' renin-angiotensin aldosterone system (RAAS) to retain sodium and water and thereby further constrict the blood vessels to maintain normal cardiac output and blood pressure; and
(c) activating hormones, including natriuretic peptides, endothelin-1, and arginine vasopressin.
Two extraordinarily rare examples of a compensatory mechanism of the body in dealing with MVD are the cases of two chihuahuas in which their MVD-affected mitral valves appear to have coapted and thereby healed themselves. See this May 2023 article. Both dogs suddenly developed symptoms of CHF and were treated on an emergency basis. They were found to have a ruptured chordae tendineae, resulting in lack of coordination (flail) of one of the two leaflets, thereby causing severe backflow of blood through the mitral valve, enlarged left atria and left ventricles, and fluid in their lungs. Treatment included injected furosemide, oxygen, and oral pimobendan, which stabilized their CHF symptoms, after which they continued to be treated with the diuretic, pimobendan, and ACE-inhibitors. In Case #1, despite the ruptured chord, the dog's heart size had reduced, and his mitral valve was functioning well enough to discontinue the furosemide and enalapril. In Case #2, after 18 months, the dog's heart no longer was enlarged and MR was substantially reduced, so the furosemide and spironolactone were discontinued. A year later, the pimobendan and benazepril were stopped. The author observed that:
"While both dogs continued to have evidence of a MV flail segment on subsequent echocardiograms, the size of the gap between the anterior and posterior MV leaflets as a result of the flail segment subjectively appeared to decrease over time."
RETURN TO TOP
Renin-angiotensin-aldosterone system (RAAS)
As noted above, one of the compensatory mechanisms to MVD is the kidneys' renin-angiotensin-aldosterone system (RAAS). It is discussed in some detail here on our Kidney Disease webpage. As for its effects upon the dog's heart, when MVD activates the RAAS, the RAAS causes the dog's body to retain fluid, and when the MVD-dog in Stage C is medicated with diuretics (to remove fluid from its lungs), the RAAS causes even more retention of fluid. RAAS even acts in the brain by binding to the hypothalamus to increase the dog's thirst and appetite for salt. Thus the RAAS, when activated by MVD and by diuretic therapy to remove fluid from the dog's lungs, works to worsen the MVD and to cause damage to the kidneys.
RETURN TO TOP
Progression of MVD
The three general stages of the progression of MVD are described here. They and other more specific categories of progression are described in more detail in our Progression & Prognosis section below.
Deformity
The first sign of progression of MVD is deformation of the natural, healthy condition of the mitral valve leaflets and chordae tendineae. Small nodules form on the edges of the valve's leaflets, particularly where the leaflets come in contact with each other. These nodules gradually enlarge to the extent of ballooning. The chords begin to thicken. The effect is to distort the leaftets to the extent that they no longer close tightly, allowing blood to backflow (regurgitate) past them. In this January 1970 article (Fig.1) and this August 1974 article, the classifications of this progression of deformity are described as:
Type 1: A few small discrete nodules in the area of contact associated with areas of diffuse opacity in the proximal portion of the valve.
Type 2: Larger nodules are evident in the area of contact, which tend to coalesce with their neighbours. Areas of diffuse opacity may be present.
Type 3: Large nodules may be seen but many have coalesced into irregular, plaque-like deformities. These lesions extend to involve the proximal portions of the chordae tendineae.
Type 4: There is gross distortion and 'ballooning' of the valve cusp, the chordae tendinae are thickened proximally.
Enlargement
In addition to the valve's leaflets and its chords, as more and more blood is able to backflow through the damaged valve, both the left atrium (LA) and the left ventricle (LV) to enlarge. This process is called cardiomegaly or dilation.
The LA is forced to enlarge (hypertrophy or dilatation) by the increase in blood coming in two directions at once and the added pressure of the blood pushing against the wall of the LA.
The LV enlarges to increase the force of its contraction, to compensate for the lessened quantity of blood the LV is intended to pump though the arteries to the body.
Apart from the mitral valve itself, the disease has severe consequences for the rest of the heart and the lungs. The increased pressure in the left atrium decreases blood flow from the lungs to the heart, resulting in congestion in the pulmonary veins, resulting in pulmonary hypertension (PH) and ultimately causing fluid, called pulmonary edema, to leak out of the capillaries into the pleural cavity of the lungs. As the left atrium enlarges, cardiac output declines.
Heart Failure
Heart failure (HF) is a condition where the heart is still working, but it can't pump enough blood to meet the dog's body's needs at the heart's normal filing pressures. Higher filing pressures can result in congestion because increased pressures in the left atrium and pulmonary veins will cause fluid from the blood vessels to leak into the lungs. This result is called congestive heart failure (CHF). See below.
HF is determined by its symptoms, which include high rates of breathing (respiratory rates), exercise intolerance, shortness of breath (dyspnea), increase in respiratory effort, and/or fainting. This form of heart faiure also is referred to as "forward heart failure".
Congestive heart failure (CHF) occurs when heart's dysfunction increases blood pressure in the pulmonary veins and capillaries, resulting in fluid seeping from the blood vessels into the lung tissues (pulmonary edema) or elsewhere (effusions). This form of heart failure also is referred to as "backward heart failure". (See the scientific definition of HF at this link.)
The left atrium usually enlarges first, followed by an enlarged left ventricle and the pulmonary veins. The heart enlargement may cause a tear in the left atrium, which usually results in immediate stoppage of blood flow. See a more detailed discussion of the progression of MVD at this section of this webpage.
To a lesser extent, cavaliers also suffer from deterioration of their tricuspid valves, called right-side heart disease or tricuspid valve disease. See this section of this webpage.
For an in-depth on-line seminar about the symptoms, diagnosis, progression, and treatment of mitral valve disease, watch Dr. Andrew Beardow, with his terrific active graphics, explain MVD.
RETURN TO TOP
Mitral valve dysplasia
Mitral valve disease is a called an "acquired" disease because it is not present at or shortly after birth and instead develops and progresses thereafter. Another category of disease of the mitral valve is "mitral valve dysplasia", which is present at or shortly after birth and therefore is congenital. This category includes malformations of the mitral valve which cause blood regurgitation back through the valve. It too results in a murmur, but at a very early age, usually before 12 months.
In a
January 2021 article, a team of Japanese veterinary clinicians
reported discovering a congenital cleft
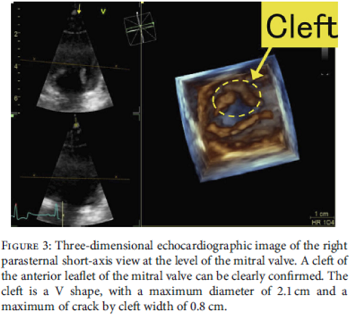 (split)
in one of the two leaflets of the mitral valve of a 2-year-old male
cavalier. The dog had mild mitral valve regurgitation of blood through
the cleft's opening. The authors used 3-D echocardiography to reveal the
position, shape, diameter, and width of the cleft's opening. (See
Figure 3 at the left.) No heart enlargement was observed, and the
dog was classified as Stage B1 of mitral valve dysplasia.
(split)
in one of the two leaflets of the mitral valve of a 2-year-old male
cavalier. The dog had mild mitral valve regurgitation of blood through
the cleft's opening. The authors used 3-D echocardiography to reveal the
position, shape, diameter, and width of the cleft's opening. (See
Figure 3 at the left.) No heart enlargement was observed, and the
dog was classified as Stage B1 of mitral valve dysplasia.
The disorder is extremely rare, and only one other case has been reported in veterinary literature -- a 2012 report of a 7-month-old English toy spaniel (King Charles spaniel). In the cavalier's case, the owners opted for continued medication with oral temocapril hydrochloride (an ACE-inhibitor) and pimobendan. The CKCS's condition did not worsen until he died of lymphocytic leukemia. Ironically, the English toy spaniel in the 2012 report also died of lymphocytic leukemia, at the age of 8.5 years.
RETURN TO TOP
Other types of heart disorders
- Tricuspid valve disease
- Innocent flow murmurs
- Systolic clicks
- Pulmonic stenosis
- Patent ductus arteriosus (PDA)
MVD is not the only type of heart disorder which cavaliers may have. Others include:
• Tricuspid valve disease: See our section on tricuspid valve disease (TVD), below.
• Innocent flow murmurs: These sounds commonly heard in 15% to 25% of healthy puppies, by using a stethoscope, are caused by turbulent blood flowing through the heart valves while the puppies' hearts are still developing. They usually are low grade murmurs heard before the 9th week, which decrease as the dog matures and are not due to any congential heart disease. Such a murmur is "likely to be innocent if it is soft (with a maximal intensity of 2 of 6), systolic, has a musical character and the point of maximal intensity is located in the region of the left cardiac base." See this November 2015 article.
• Systolic clicks: These are sounds heard with a stethoscope placed over the mitral valve of cavaliers not yet diagnosed with MVD murmurs. Veterinary cardiologist Dr. James Buchanan stated in 1998 that "systolic clicks occur twenty-five times more frequently in cavaliers than other breeds and may be a precursor to a murmur showing up a few years later ." Cardiologist Dr. Michele Borgarelli has stated in 2020 that such a click sound may be caused by vibration of the mitral valve leaflets and tensing of the valve's chordae tendineae as the leaflets slighty prolapse.
• Pulmonic stenosis (pulmonary valve stenosis): This is a fairly common congenital heart defect in cavaliers. It is characterized by the narrowing and obstruction of blood through the heart's pulmonary valve, which connects the pulmonary artery to the right ventricle chamber. Read more details about this disorder here.
• Patent ductus arteriosus (PDA): This is the most common congenital cardiovascular abnormality in dogs. Cavaliers have been shown to have a "high prevalence" for PDA. It occurs when a temporary blood vessel -- the arterial canal -- which is used to bypass the fetus's undeveloped lungs in the womb and allows blood to pass from the right side of the heart to the left, fails to seal within a week after birth. Normally, this vessel will begin to seal once the puppy begins breathing. PDA compromises the circulation of blood through the heart. It is believed to be inherited as a polygenic threshold trait with a high rate of heritability in some breeds and is considered to be hereditary in the CKCS. Read more details about this disorder here.
RETURN TO TOP
Symptoms
- Internal symptoms
- Visible symptoms
- Coughing
- Later symptoms
- Exercise intolerance and loss of muscle mass (cachexia)
- Syncope or pre-syncope
- Near death signs
- Assessment of quality of life
Until the MVD-affected dog reaches "heart failure" (HF or CHF),
there usually are no visible signs or symptoms related to
 the MVD. A murmur
and an
enlarging heart, which come before heart failure, are not considered symptoms,
because they cannot be outwardly visible. A murmur is listened to with a
stethoscope (auscultation), and enlargement is determined by comparing
measurements taken from sets of x-rays or echocardiogram scans, none of
which are observable externally. Prior to the dog reaching the MVD stage
called heart failure, these signs -- murmurs and enlargement -- are
referred to as "asymptomatic" indications of MVD.
the MVD. A murmur
and an
enlarging heart, which come before heart failure, are not considered symptoms,
because they cannot be outwardly visible. A murmur is listened to with a
stethoscope (auscultation), and enlargement is determined by comparing
measurements taken from sets of x-rays or echocardiogram scans, none of
which are observable externally. Prior to the dog reaching the MVD stage
called heart failure, these signs -- murmurs and enlargement -- are
referred to as "asymptomatic" indications of MVD.
"Heart failure" in this context does not mean that the dog's heart stops beating. It means instead that the dog shows signs that the heart is not functioning at full capacity and appears to be declining in its ability to pump blood throughout the dog's systems. "Congestive heart failure" (CHF) means that the dog's (pulmonary) veins in its lungs are congested with blood which no longer is flowing properly, causing the blood vessels to leak and release fluids into the lung tissue.
(NOTE: Veterinarians will tell us that dogs do not have "symptoms" but instead have "signs". But, for us laymen, the word "signs" can be confusing because of its different meanings. So, for us, "symptoms" it is.)
RETURN TO TOP
• internal symptoms
• mitral regurgitation
As discussed in more detail below,
mitral
regurgitation (MR) is blood moving backwards through the
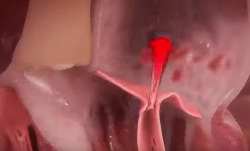 mitral valve from
the left ventricle to the left atrium. This backflow usually makes a
soft sound, called a mumur, that can be heard with a stethoscope
(auscultation). In some milder cases, the sound of the backflow cannot
be heard, but it can be observed during echocardiograph scans.
mitral valve from
the left ventricle to the left atrium. This backflow usually makes a
soft sound, called a mumur, that can be heard with a stethoscope
(auscultation). In some milder cases, the sound of the backflow cannot
be heard, but it can be observed during echocardiograph scans.
(In the image at right, the red spurt of blood is shooting upward and backward, from the left ventricle, through the not-fully-closed mitral valve, back into the left atrium.)
The more severe the MR, the greater likelihood the MVD will progress more rapidly. The severity of MR is calculated as a percentage, using echocardiography, particularly color flow Doppler. MR is classified as:
• No MR (0%)
• Mild MR (under 20%)
• Moderate MR (20% to 50%)
• Severe MR (over 50%)
See this extended discussion of mitral regurgitation below, for more information.
• murmur
The mitral regurgitation produces a sound as tubulent blood flows backwards through the mitral valve. This is called a heart murmur, heard by means of a stethoscope. It is the first indication that the dog may have mitral valve disease (MVD). MVD murmurs are graded according to their loudness, from the softest sound, a Grade 1, to the loudest, a Grade 6. In this July 2025 article, the authors state:
"The presence of a murmur increases the likelihood of MMVD being pre sent, and the absence of a murmur all but rules out MMVD".
More details about MVD murmurs are discussed below at this link.
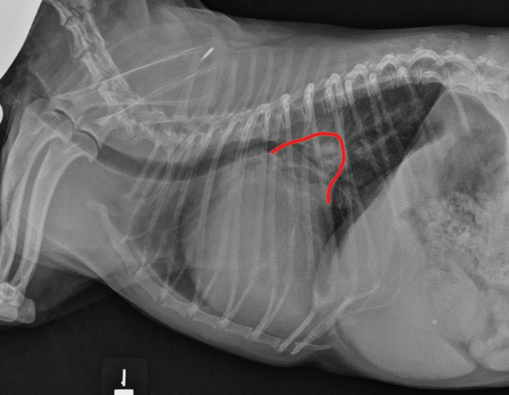
• enlargement of the heart's left side
As noted above, what is not visible outwardly, before the dog reaches heart failure, is enlargement of the left chambers of the heart. As greater quantities of blood leak through the damaged mitral valve from the left ventricle back into the left atrium, the thin-walled atrium gradually begins to swell and enlarge (see x-ray image of a severely enlarged left atrium, outlined in red, above) -- called remodeling or cardiomegaly or dilation -- to accommodate the overload of blood, and there is a reduction in the ability of the left ventricle to provide sufficient blood to meet the demands of the rest of the body. The heart then has to pump harder and faster, to meet those demands. The shut-down of the distant blood vessels also has the effect of causing the left ventricle to beat against a higher resistance, causing another increase in mitral valve leakage.
• fluid in the lungs
Heart enlargement due to mitral regurgitation also has been shown to cause cardiac arrhythmias. See this January 2019 article.
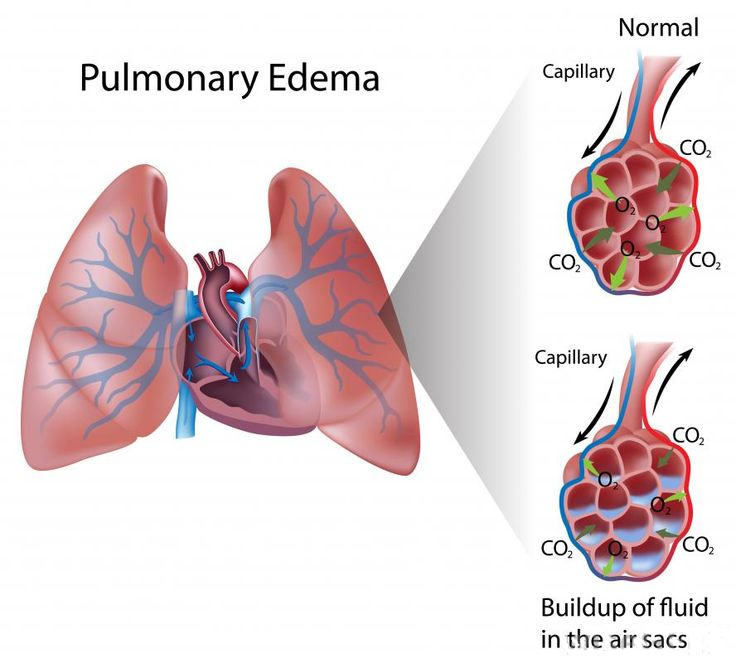 Also, the overload of blood in the left atrium creates increased
pressure back into the pulmonary veins, which drain into the left atrium
from the lungs. When a critical pressure is reached, flooding of the
lungs can occur, causing pulmonary edema.
Also, the overload of blood in the left atrium creates increased
pressure back into the pulmonary veins, which drain into the left atrium
from the lungs. When a critical pressure is reached, flooding of the
lungs can occur, causing pulmonary edema.
Edema results when blood vessels leak and release fluids into nearby tissues. The extra fluid accumulates, causing the tissue to swell. Edema is a normal response of the body to inflammation or injury. When the heart weakens and pumps blood less effectively, fluids can slowly build up, creating edema. If fluid buildup occurs rapidly due to damage to the left side of the heart, fluid in the lungs -- pulmonary edema -- can develop. If there is heart failure of the right side of the heart, edema can develop in the abdomen.
For an in-depth on-line seminar about the symptoms, diagnosis, progression, and treatment of mitral valve disease, watch Dr. Andrew Beardow, with his terrific active graphics, explain MVD.
RETURN TO TOP
• visible symptoms of heart failure:
The closer the MVD gets to heart failure, the more rapid the dog's breathing becomes while asleep. Usually more than 30+ breaths per minute while sound asleep or resting indicates the onset of congestive heart failure, due to the build-up of fluid in the dog's lungs (pulmonary edema). Breathlessness also is a most common sign, starting as excessive panting on exercise. This shortness of breath is called "dyspnea"; the rapid breathing is called "tachypnea".
A dog with fluid in its lungs may have difficulty finding a comfortable position when lying down.
Note that some cardiologsists refer to two categories of heart failure -- the traditional symptomatic one which they call the "decompensated phase", and an earlier "compensatory phase" which displays no symptoms. See this January 2018 article.
• coughing is not a symptom of CHF
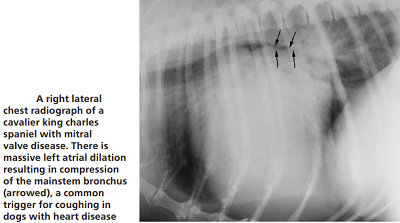 Most MVD-affected dogs with a cough are not yet in heart
failure. However, many veterinarians associate a dry, hacking cough with an enlarged
heart due to MVD or even to heart failure. They call it a "cardiac
cough" and attribute it to either the enlarged left atrium conpresising
against the dog's bronchus or trachea (see photo at right), or, fluid build-up in the lungs due to CHF
(pulmonary edema). In most all cases, however, the term cardiac cough is
a misnoner because the true cause of the cough probably is respiratory
related and completely independent of any heart disorder.
Most MVD-affected dogs with a cough are not yet in heart
failure. However, many veterinarians associate a dry, hacking cough with an enlarged
heart due to MVD or even to heart failure. They call it a "cardiac
cough" and attribute it to either the enlarged left atrium conpresising
against the dog's bronchus or trachea (see photo at right), or, fluid build-up in the lungs due to CHF
(pulmonary edema). In most all cases, however, the term cardiac cough is
a misnoner because the true cause of the cough probably is respiratory
related and completely independent of any heart disorder.
One of the most common -- and potentially dangerous -- misjudgments some veterinarians tend to make is to assume that such a cough indicates the onset of CHF and the need for immediate treatment with a diuretic. Cardiologist Dr. Michele Borgarelli has warned:
"It should be stressed that cough is a general clinical sign of respiratory disease and its presence in a dog with a murmur is not an indicator for starting treatment for CHF."
In this July 2025 article, the authors concur, stating: "Stage B2 dogs may show signs of coughing, which appears to be more likely in dogs with cardiac enlargement."
The cough could be due to a combination of factors, which include the enlarged left atrium of the heart pressing against and compressing the left mainstem bronchus or trachea, but more likely to airway disorders independent of any relationship with the heart, such as bronchomalacia and airway inflammation (possibly with the large left atrium merely highlighting the bronchial narrowing). It may even cause the trachea to collapse*. However, if the dog coughs up a pink-tinged fluid, it would indicate that may have very severe pulmonary edema which is filling the airways.
* Trachea collapse also may be due to Brachycephalic airway obstruction syndrome (BAOS).
In a March 2012 article, USA researchers found no association between left atrial enlargement (LAE) and bronchial collapse in MVD-affected dogs with moderate-to-severe LAE and chronic coughing, but they did find that airway inflammation is common in dogs with airway collapse.
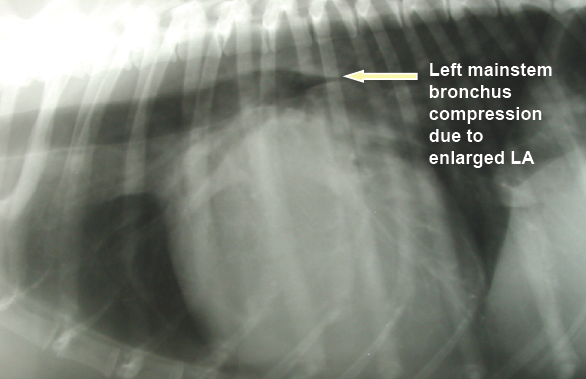
In a January 2019 article, a pair of experts concluded that a cough in the absence of rapid and labored breathing would indicate that it is due mainly to a respiratory disease rather than a cardiac disease. Coughing is a hallmark sign of bronchitis. Dogs with severe pulmonary edema can cough, but coughing is much more common with primary lung disease. By severe pulmonary edema, they mean that the fluid has completely filled the lungs and also has started to fill the upper airway passages, as well. (The x-ray below shows the heart's enlarged left atrium impinging upon the left main bronchus.).
In an April 2021 article, a team of French and US investigators used computed tomography (CT) to study the association between left atrium enlargement causing bronchial narrowing in 21 coughing dogs (2 cavaliers -- 9,5%) diagnosed with heart murmurs due to MVD. They measured each dog using chest x-rays, echocardiography, and chest CT scans. They calculated bronchial narrowing using the bronchial-to-aorta ratio. They report finding "significant narrowing" of the bronchi in the coughing dogs, compared to 10 control group non-coughing, healthy dogs. They noted that as the left atrium size (LA/Ao) and vertebral heart size (VHS) increased, the diameter of all left-sided and most right-sided bronchi decreased. The LA/Ao range was from 1.5 to 3.5, and three dogs (14%) had a LA/Ao ratio below 1.6.
In an October 2025 article, veterinary cardiologists Mark Rishniw, Michele Borgarelli, Luca Ferasin, and Giulio Menciotti examined the effect of left atrial (LA) size and presence or absence of congestive heart failure (CHF) in small-breed dogs with mitral valve disease on the probability of coughing. They hypothesized that coughing probability would increase with increasing left atrial enlargement but not with CHF. They examined the records of 352 dogs with varying degrees of LA enlargement, up to severe enlargement, which the categorized as having a left atrium-to-aortic root ratio (LA:Ao) greater than 2.29. They report finding that only severe LA enlargement resulted in an increased probability of coughing in MVD-affected dogs. They found that dogs lesser degrees of LA enlargement had similar probabilities of coughing as dogs without any enlargement. They further found that CHF was not an independent predictor of coughing. They concluded that they found no evidence that CHF contributes to or increases the probability of coughing in dogs with MVD and that severe LA enlargement does increase this probability. They urged that clinicians should not include coughing (or its absence) when considering whether dogs with MVD have CHF or not. Specifically, they stated:
"Our study should dispel the idea that pulmonary edema causes coughing in dogs with mitral valve disease -- indeed, clinicians should ignore the presence of coughing when making a diagnosis of CHF in dogs with mitral valve disease. Specifically, clinicians cannot make the assumption that a coughing dog with a murmur has CHF -- a diagnostic error that clinicians in first-opinion practice often make. While some dogs with CHF developed a cough only when they presented with CHF, the logistic regression analysis did not identify CHF as an independent predictor of coughing. Furthermore, given that the pattern of probabilities associated with coughing in these dogs mirrored that of dogs with subclinical MMVD, our findings suggested that these dogs quite possibly developed a cough because of their left atrial enlargement, and not the development of CHF per se."
So if a dog has had a cough for months and unchanged and the dog is not being treated with a diuretic, the cough is very unlikely due to heart failure. Some cardiologists may prescribe a bronchial dilator (bronchodilator), such as a methylxanthine, for example, aminophylline, millophyline, oxtriphylline, theophylline* (Corvental, Nuelin, Apo-Theo-LA, Theo-Dur), or terbutaline (Brethine, Bricanyl) which are human grade prescription medications which relax and open air passages in the lungs, making breathing easier.** A narcotic, hydrocodone bitartrate with homatropine MBr or guaifenesin (Hycodan, Hydromet, Tussigon, Mycodone), or butorphanol tartrate (Torbutrol, Stadol, Torbugesic) or diphenoxylate hydrochloride and atropine sulfate (Logen, Lomotil, Lonox, Lofenoxal), primarily an anti-diarrheal but also a cough suppresssant, may be prescribed to suppress the coughing by affecting the brain's cough centers. Budesonide inhalation (Pulmicort Flexhaler, Pulmicort Respules) is a steroid inhalation suspension prescribed to prevent asthma attacks in humans.
* Theophylline is a
PDE inhibitor which should not be given concurrently with pimobendan, which
is another PDE inhibitor, unless the combination of those two drugs is
carefully balanced.
** Fluoroquinolone antibiotics should not be given concurrently with any
methylxanthines.
There is a possibility that a particular angiotensin-converting enzyme inhibitor (ACE-inhibitor) may have cough-suppressant effects on MVD-affected dogs with a cardiac cough. In an August 2018 article, a team of Japanese researchers tested the ACE-inhibitor alacepril on 36 dogs, including four cavalier King Charles spaniels, which were in Stage B2 of mitral valve disease (having an MVD-murmur and an enlarged heart but prior to heart failure) and all displaying an MVD-related cough. They primarily were testing the cough-suppression efficacy of alacepril over a period of four weeks. They report finding that alacepril resolved or lessened the cough in 20 (55.6%) of the dogs and had no cough-suppressant effect upon the remaining 16 (44.4%). They observe that alacepril is among a sub-group of ACE-inhibitors (including captopril and zofenopril) which contain sulfhydryl, which may confer properties additional to ACE inhibition and which may explain the cough-suppressant qualities.
RETURN TO TOP
• later symptoms of heart failure:
Once congestive heart failure has been diagnosed, look for these additional signs: exercise intolerance, lack of appetite, restlessness at night, weight loss, and fainting (called syncope). As breathing difficulties become more severe, the dog may sit or stand, holding its elbows away from the chest, and it may be reluctant to sit down. In some cases, any of these symptoms may appear even before the onset of congestive heart failure.
• exercise intolerance and loss of lean muscle mass (cardiac cachexia)
The inability to withstand normal levels of exercise is a classic sign of the progression of the MVD. This is attributed to a variety of systemic changes which take place in the dog's body, including pulmonary hypertension and cardiac cachexia.
Cardiac cachexia is the loss of lean muscle mass, especially in the hind quarters, which has been found primarily in MVD-affected dogs in congestive heart failure (CHF). The loss of body mass in these dogs has deleterious effects upon the dogs' strength and immune functions. Research has shown that loss of muscle mass is due to:
• an insufficiency of oxygen getting to the dog's muscles;
• reduction in capillary length density, leading to inadequate capillary blood per unit volume of skeletal muscle;
• reduced mitochondria* mass in the skeletal muscles, resulting in more limited oxidative capacity; and
• transformation of muscle fibers from type I (slow twitch) to type II (fast twitch), particularly type IIB, which possess less oxidative capacity than type IIA or type I fibers. Type IIB fibers have a low aerobic potential, and are easily fatigued.
*Mitochondria in each cell of the dog's body are organelles which take in nutrients, break them down, and create energy rich molecules for the cell.
For more information about the effects of heart failure upon skeletal muscles, see this November 1989 article and this May 1992 article, both of which are about human patients, and this May 2017 article, specifically pertaining to dogs in heart failure. In an April 2004 article, Danish cardiologists found in a study of 109 cavaliers that:
"... a weak femoral [hind leg] artery pulse is a common finding in CKCS and Dachshunds, and that the pulse strength in any one dog is reasonably reproducible. Pulse strength was found to be inversely related to heart rate, degree of obesity and MVP [mitral valve prolapse] severity. In addition, femoral artery pulse strength decreased with age. The weak femoral artery pulses appear to reflect a decrease in diastolic artery diameter and/or systolic distension associated with a local decrease in blood flow, not artery occlusion or changes in pulse pressure and stroke volume."
See our discussion of treating exercise intolerance and loss of muscle mass in our Treatment -- Stage C -- heart failure subsection, for more information.
The dog may also display episodic weakness of the hindquarters, ataxia (lack of coordination), involuntary shaking of a hind leg, or collapse (presyncope), or combined with loss of consciousness (syncope), due to a sudden decline in blood flow to the brain. See Syncope for a discussion of this disorder and its causes.
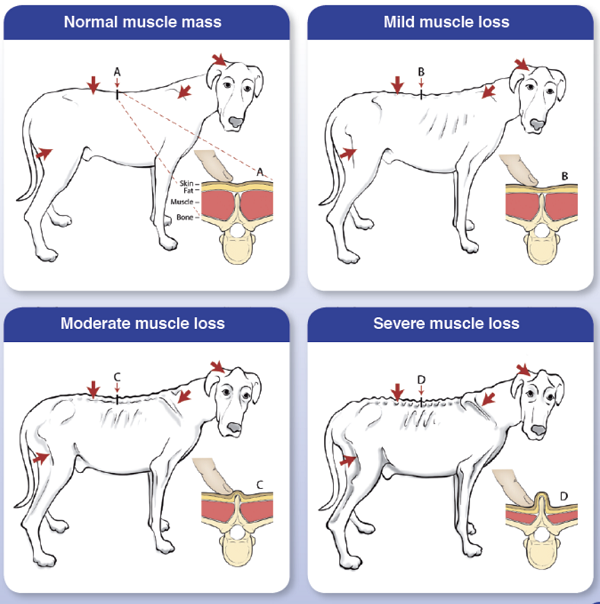
Cardiac cachexia also includes a loss of appetite, resulting in possibly severe weight loss. Over half of all dogs in CHF due to MVD will develop cardiac cachexia. In a July 2019 article, a team of researchers at Tufts University's Cummings veterinary school examined 218 MVD-affected dogs in heart failure (CHF), including 20 cavalier King Charles spaniels, to determine the role of muscle loss -- called cardiac cachexia -- in life expectance of the dogs. Cardiac cachexia is defined as the loss of muscle mass associated with CHF. A second definition is the rapid loss of overall weight (>5%) within 12 months. They found that dogs with cachexia had a significantly shorter survival time compared with dogs without cachexia. They also report that cachexia is common in dogs with CHF. They emphasize "the importance of preventing, diagnosing, and treating muscle loss in dogs with CHF". Tufts provides this chart (below) to detect muscle loss in MVD-affected dogs in CHF.
RETURN TO TOP
• syncope or pre-syncope
Fainting or the loss of consciousness in MVD-affected dogs in the late stages of the disease is called syncope; absent the loss of consciousness, it is called presyncope. For more information, see our Syncope webpage.
RETURN TO TOP
• near death signs
As the cavalier nears death from MVD, often the dog will display a severe air hunger and uses all of its remaining energy just trying to breathe.
RETURN TO TOP
• assessment of quality of life
In a 2005 report, cardiac researchers at Tufts University's Cummings School of Veterinary Medicine devised a survey that may prove to be similarly useful in evaluating the quality of life for dogs with heart disease. Known as "FETCH" (Functional Evaluation of Cardiac Health), the survey asks the dogs' owners to rank aspects of their dog's health on a scale of 0 to 5. Veterinarians are then able to assess the animal's perceived quality of life, which may inform decisions about treatment, nutrition or even euthanasia. Researchers found that the FETCH scores correlated well to the International Small Animal Cardiac Health Council (ISACHC) classification for disease severity. See our in depth discussion of this quality-of-life questionnaire, below.
RETURN TO TOP
Diagnosis
- Auscultation (stethoscope)
- X-rays (radiography)
- Respiratory rates
- Heart beat rates
- Ultrasound (echocardiography)
- Cardiac magnetic resonance imaging (cMRI)
- Computed tomography (CT)
- Electrocardiography (ECG or EKG)
- Artificial intelligence
- Cardiac catheterization
- Mass spectrometry
- Natriuretic peptides tests (ANP and BNP)
- Other cardiac biomarkers
- Quality of life questionnaire
-- auscultation (stethoscope)
- Heart murmur grades
- Clicks
- Fluid in the lungs
- Other types of heart murmurs
- Digital stethoscope -- Phonocardiogram
The sound of tubulent blood flow over the mitral valve, called a
heart murmur, heard by means of a
 stethoscope, is the first indication
that the dog may have mitral valve disease (MVD).
stethoscope, is the first indication
that the dog may have mitral valve disease (MVD).
Not all heart murmurs are due to blood backflowing through the mitral valve. The precise location of the source of the sound is an important aspect of diagnosing MVD by listening with a stethoscope. That location is at the left apex and during the systolic phase of the heart beat. If the murmur is not coming from that location and at the time, then it is less likely an indication of MVD and more likely another heart disorder. See Mitral Valve Dysplasia and Other Types of Heart Disorders above for details of other types of heart murmurs. In this July 2025 article, the authors state:
"It [MVD] is by far the most likely cause of a murmur detected for the first time in an adult small breed dog (<15 kg) more than 5 year old, especially if the murmur is systolic and detectable at the left heart apex."
Cavaliers should be screened for heart murmurs annually, beginning at age one year. Once MVD is detected, its progression can be monitored with stethoscopic examinations (auscultations), x-rays, respiratory rates (breaths per minute while resting or asleep), echocardiograms, and color Doppler echocardiograms. If a cavalier's heart murmur is first detected by a general practice veterinarian, it should be confirmed within 3 to 6 months by a specialist, preferably a board certified veterinary cardiologist. If it still is detected, the dog has MVD.
Incidentally, we also recommend that as soon as a murmur first is detected in any cavalier -- or even before a murmur is detected in a full grown CKCS -- that the owner obtain a set of chest x-rays (thoracic radiographs) to serve as a baseline set for comparison with later x-rays to determine if any enlargement of the heart has occurred in the interim.
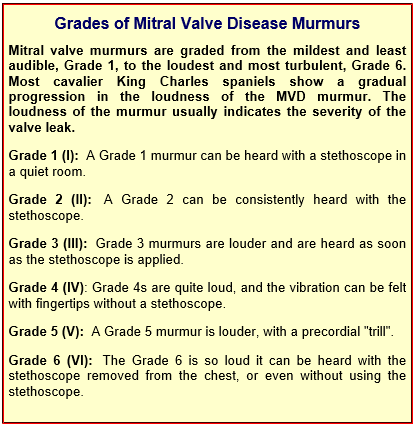
Heart murmur grades
The first indication of MVD which is detectable apart from an echocardiograph (ultrasound) examination, is the presence of a soft whistling sound, called a "murmur", which can be heard by a veterinarian using a stethoscope, which is called auscultation. The murmur sound is caused by the turbulent flow of blood jetting backwards through the damaged leaflets of the mitral valve from the left ventricle, into the left atrium. This reverse flow of blood through the mitral valve is called mitral regurgitation (MR). (Photo above is of veterinary cardiologist Dr. Michele Borgarelli auscultating a cavalier's heart with a stethoscope.)
As simple a device as the stethoscope may seem to be, many cardiologists consider that auscultation is the best screening test there is for the identification of the presence of mitral valve regurgitation. The loudness of the murmur determines its "grade". (See the "Grades of Mitral Valve Disease Murmurs" below.) An alternative method of grading murmurs, discussed in this November 2014 article, provides:
• Soft (quieter than heart sounds) = Grades I and II
• Moderate (as loud as heart sounds) = Grade III
• Loud (louder than hart sounds) = Grade IV
• Thrilling (very loud, heard with stethoscope removed from chest) = Grades V and VI
MR usually is classified as mild, moderate, or severe. However, there is not necessarily a direct correlation between the loudness of the murmur and the amount of blood flowing backward through the mitral valve. While soft mitral murmurs -- Grades 1 or 2 -- almost always indicate mild MR, once the murmur edges upwards from there, there often is no correlation between the murmur's grade and the degree of mitral regurgitation. However, However, two studies -- November 2014 and December 2018 -- have concluded that thrilling murmurs (grades V and VI) are associated with more severe MVD.
Ask the cardiologist to use this standardized report form. A list of upcoming heart testing examination clinics is on our Health Clinic webpage.
Also, ask the cardiologist about the American College of Veterinary Internal Medicine (ACVIM) Registry of Cardiac Health (ARCH), a registry and database for canine hearts examined by board certified cardiologists. See the details on the ARCH website.
In a 2009 "Consensus Statement" published by a panel of the board certified veterinary cardiologists of the American College of Veterinary Internal Medicine (ACVIM), they state:
"Consensus recommendations:
"Small breed dogs, including breeds with known predisposition to develop CVHD [chronic valvular heart disease] (e.g., Cavalier King Charles Spaniels, Dachshunds, Miniature and Toy Poodles) should undergo regular evaluations (yearly auscultation by the family veterinarian) as part of routine health care.
"Owners of breeding dogs or those at especially high risk, such as Cavalier King Charles Spaniels, may choose to participate in yearly screening events at dog shows or other events sponsored by their breed association or kennel club and conducted by board-certified cardiologists participating in an ACVIM-approved disease registry."
The accuracy of auscultations can be very subjective, and in some cases, cavaliers with no detected murmur may in fact have a significant stage of MVD. For example, in an October 2023 article, 27 cavaliers had no heart murmur, but upon echocardiographic examination:
"Of these 27 dogs, two had moderate MR [mitral regurgitation] on their echocardiogram, 12 had mild MR, and 13 had none or trivial MR but had mitral valve remodeling consistent with MMVD such as mitral valve prolapse and/or thickening. In CKCS that were stage B2, 91.1% had a heart murmur that was a grade 4/6 or louder." (Emphasis added.)
For an in-depth on-line seminar about the symptoms, diagnosis, progression, and treatment of mitral valve disease, watch Dr. Andrew Beardow, with his terrific active graphics, explain MVD.
Listen to the sound of a Grade 3 MVD heart murmur here. Other levels of murmurs may be heard here.
Our Blog: "So your cavalier has a heart murmur. What do you do next?"
In her June 2025 article, Prof. Melanie Hezzell stateds that, "in a middle-aged to older small-breed dog it is reasonable to assume that MMVD is the most likely cause of a murmur of mitral regurgitation."
Clicks
Even if the veterinarian does not hear a murmur, he might report hearing a "systolic click" when he examines the dog with his stethoscope. Veterinary cardiologist Dr. James Buchanan stated in 1998 that "systolic clicks occur twenty-five times more frequently in cavaliers than other breeds and may be a precursor to a murmur showing up a few years later ." Cardiologist Dr. Michele Borgarelli has stated in 2020 that such a click sound may be caused by vibration of the mitral valve leaflets and tensing of the valve's chordae tendineae as the leaflets slighty prolapse.
Fluid in the lungs
Veterinarians also use the stethoscope to listen to the dog's lungs if congestive heart failure (CHF) is suspected. One of the signs of CHF is fluid in the lungs (called edema), and that fluid tends to make crackling noises when heard with a stethoscope. Cardiologist Dr. Dave Dickson has described the sound of fluid in the lungs as "like Velcro unpeeling". However, hearing those sounds is not definitive of CHF, and other devices should be used to confirm edema, including high sleeping respiratory rates, chest x-rays, and lung ultrasound.
Other types of heart murmurs
MVD is not the only type of heart disorder which may produce heart murmurs in cavaliers. Others include:
• Innocent flow murmurs: These sounds heard in puppies, by using a stethoscope, are caused by turbulent blood flowing through the heart valves while the puppies' hearts are still developing. They usually are low grade murmurs (Grade 1 or 2) which decrease as the dog matures and are not due to any congenital heart disease. If a high grade murmur is detected early, say at 6 weeks, then it being due to a congentital disoder is more likely, and the dog should be examined by a cardiologist using ultrasound (echocardiography).
• Pulmonic stenosis (pulmonary valve stenosis): This is a fairly common congenital heart defect in cavaliers. It is characterized by the narrowing and obstruction of blood through the heart's pulmonary valve, which connects the pulmonary artery to the right ventricle chamber. Read more details about this disorder here.
• Patent ductus arteriosus (PDA): This is the most common congenital cardiovascular abnormality in dogs. Cavaliers have been shown to have a "high prevalence" for PDA. It occurs when a temporary blood vessel -- the arterial canal -- which is used to bypass the fetus's undeveloped lungs in the womb and allows blood to pass from the right side of the heart to the left, fails to seal within a week after birth. Normally, this vessel will begin to seal once the puppy begins breathing. PDA compromises the circulation of blood through the heart. It is believed to be inherited as a polygenic threshold trait with a high rate of heritability in some breeds and is considered to be hereditary in the CKCS. Read more details about this disorder here.
• Quadricuspid aortic valve: The aortic valve is the gateway for blood to exit out of the ventricle and pass through the aorta artery for the body to receive oxygenated blood. Normal aortic valves have three equally sized leaflets, called (1) the right coronary cusp, (2) the left coronary cusp, and (3) the non-coronary cusp. Quadrisupid aortic valve (QAV) is a rare congenital heart disease in which a fourth cusp develops, resulting in regurgitation through the aortic valve, causing the sound of a murmur detected by auscultation. Read more details about this disorder here.
Digital stethoscope -- Phonocardiogram
A digital stethoscope is a standard stethoscope with the added
capability of converting the acoustical sound to electronic signals,
which then are transmitted to a computer for further amplification.
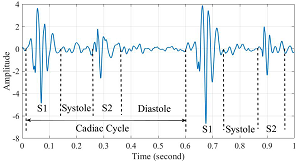
A phonocardiogram (PCG) is a graphic recording of heart sounds detected using a digital stethoscope. The phonocardiogram appears as a digital image on a computer screen. Phonocardiograms can produce sound recordings of the heart beats and murmurs, as well as display images showing "waveforms" (see image at right) which enable the clinician to distinguish between normal heart beats and abnormal sounds, such as murmurs, in a more objective manner than the clinician listening to those sounds using a non-electronic (acoustical) stethoscope.
Phonocardiograms are used to detect heart murmurs and to distinguish between mitral valve murmurs due to mitral regurgitation and others, including innocent flow murmurs, aortic regurgitation, aortic and mitral stenosis, and valvular lesions.
In a May 2025 article, South Korean researchers examined 460 dogs diagnosed with mitral valve disease (MVD) using digital stethoscopes (WP-100 digital stethoscope), phonocardiogram signals, and three different deep learning programs -- convolutional neural network (CNN6), patch-mix audio spectrogram transformer (PaSST), and residual neural network (ResNET38). They focused upon the severity of mitral regurgitation (MR) -- mild, moderate, severe -- and compared the data obtained using stethoscopes to echocardiograms of each dog and their MINE scores. They report finding that "the CNN6-Fbank model achieved an accuracy of 94.12%, specificity of 97.30%, sensitivity of 94.12%, precision of 92.63%, and F1 score of 93.32%, outperforming the PaSST and ResNet38 models overall and demonstrating robust performance across most metrics." They concluded: "Deep learning models, particularly CNN6, can effectively assess MR severity in dogs with MMVD using digital stethoscope recordings. This approach provides a rapid, noninvasive, and reliable adjunct to echocardiography, potentially enhancing diagnosis and outcomes. Future studies should focus on broader clinical validation and real-time application of this technology."
RETURN TO TOP
-- x-rays (radiography)
- Using radiography to diagnose heart enlargement
- VHS measurements to detect enlargement
- Heart-to-single-vertebra ratio
- VLAS measurements of left atrium
- Modified-Vertebral Left Atrial Size (M-VLAS)
- Radiographic left atrial dimension (RLAD)
- Vertebral heart area ratio (VHAR)
- Left atrial width (LAwidth)
- Subjective analysis of x-rays of MVD-affected hearts
- Left atrium enlargement (LAE)
- Left ventricle enlargement
- Quality and standardization of x-rays
- Baseline and periodic x-rays
- Diagnosing pulmonary edema and heart failure
- Angiography and Angiocardiography
- Cardiothoracic Ratio (CTR)
- Manubrium heart score (MHS)
- Thoracic inlet heart score
- Dexmedetomidine
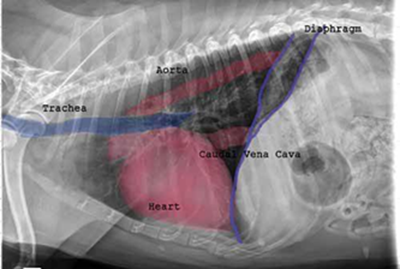 Radiography (x-ray) is used to determine if the heart is enlarged
(particularly the left atrium and left ventricle), if the veins from the
lungs to the heart are distended, or if fluid is beginning to develop in the
lungs.* X-rays also will show any enlargement of
the pulmonary vein, a classic symptom of congestive heart failure (CHF).
Radiography (x-ray) is used to determine if the heart is enlarged
(particularly the left atrium and left ventricle), if the veins from the
lungs to the heart are distended, or if fluid is beginning to develop in the
lungs.* X-rays also will show any enlargement of
the pulmonary vein, a classic symptom of congestive heart failure (CHF).
*X-ray is the best diagnostic device for detecting fluid in the dog's lungs. See below.
Once a mitral valve murmur is detected by auscultation, annual x-rays are very useful in charting the progression of the disease. The 2009 ACVIM "Consensus Statement" on degenerative MVD recommends baseline thoracic radiography for dogs with a new mitral valve murmur, then annually thereafter. However, if auscultation by stethoscope indicates that a murmur has remained mild -- Grade 1 or 2 -- and has not progressed in loudness since the murmur was first detected, a set of follow-up x-rays the next year usually is not necessary.
RETURN TO TOP
• Using radiography to diagnose heart enlargement
Mild to moderate heart enlargement indicates mild to moderate progression of mitral regurgitation (MR), with the heart compensating for the effects of mitral regurgitation by enlarging. Usually the dog displays no outward signs or symptoms of MVD when moderate to severe heart enlargement develops, until the dog reaches the stage of heart failure.
In a May 2020 article, a team of Korean veterinary investigators compared chest (thoracic) x-rays of MVD-affected dogs both at full inspiration (inhalation) and full expiration (exhalation) when measuring the apparent sizes of the dogs' hearts. The study included 20 beagles with normal-sized hearts and 100 MVD-affected dogs of unidentified breeds. They measured the vertebral heart scale (VHS), vertebral left atrial size (VLAS), position of the trachea, and bulging of the left atrium (LA), and appearance of the left ventricle (LV). They compared this data to each dog's left atrium to aorta echocardiographic ratio (LA/Ao). They concluded:
"In dogs with MR [mitral regurgitation], LA enlargement was subjectively more apparent when thoracic radiography was taken during the expiratory phase compared to the inspiratory phase. These findings supported the use of both inspiratory and expiratory radiographs for assessment of left heart enlargement in dogs with MR, although measures of LA enlargement in dogs with MR can be overestimated in expiratory radiographs."
RETURN TO TOP
• VHS measurements to detect enlargement
Cardiologists use x-rays to evaluate the size and shape of the heart in
order to assess the severity of MVD.
The Vertebral Heart
Size or Scale or Sum (VHS) is an objective means of measuring
heart size.
 As
the x-ray image above shows, using calipers, they
measure the length (from the apex to the bottom of the left mainstem) and width
(at
its widest point perpendicular to the lemgth measurement) of the heart
on a lateral x-ray and compare those dimensions
to the number of veterbrae from T4 to T12, to calculate the VHS value. Since
the dog's own vertebrae are used for comparison, each VHS value is
normalized to the dog's overall body size.
As
the x-ray image above shows, using calipers, they
measure the length (from the apex to the bottom of the left mainstem) and width
(at
its widest point perpendicular to the lemgth measurement) of the heart
on a lateral x-ray and compare those dimensions
to the number of veterbrae from T4 to T12, to calculate the VHS value. Since
the dog's own vertebrae are used for comparison, each VHS value is
normalized to the dog's overall body size.
 See
this
YouTube video for details. A diagram showing how the VHS is calculated
is
here. This is called the Buchanan VHS method, devised
by Dr. James W.
Buchanan, a pioneer in the research of MVD in cavaliers, in
1995. See his
1995 article.
See
this
YouTube video for details. A diagram showing how the VHS is calculated
is
here. This is called the Buchanan VHS method, devised
by Dr. James W.
Buchanan, a pioneer in the research of MVD in cavaliers, in
1995. See his
1995 article.
The VHS is not intended to diagnose CHF. It's purpose is to enable veterinarians to more accurately determine enlargement of the heart (called cardiomegaly or dilation) and the progression at which the enlargement is occurring, which usually is due to MVD. However, since MVD normally initially causes only the left atrium (LA) to enlarge, the VHS method is not precise enough to measure only the size of the LA. See for example, the x-ray at left, which shows that the VHS measurements entirely miss the bulbous enlarged LA in the upper right corner of the heart. (Image from Hezzell, 2018.)
The VHS method is most effective when it is used to compare two x-rays of the same dog's heart, taken over time, to see if the later x-ray's VHS value is higher than the earlier one, thus indicating that an enlargement of the heart has occurred. Therefore, once an MVD murmur is first detected, it is advisable to obtain an initial set of chest x-rays (called a "baseline" set) for comparison with subsequent x-rays once progression of the MVD is suspected.
An increase in VHS value of >0.1 per month over six months or more, in combination with a VLAS value >3.0, (see VLAS below) is evidence that the patient's heart has enlarged enough to classify it in Stage B2.
Some veterinary cardiologists have used the VHS method to try to determine breed-specific and even species-wide ranges of heart sizes of dogs with healthy (non-MVD) hearts, rather than just to compare VHS values in periodic x-rays of the same dog. There are published breed-specific studies of ranges of VHS values for dogs' healthy hearts for dozens of breeds.
The VHS values of cavaliers which do not have any heart enlargement can vary widely. In a 2001 study of 20 CKCSs and a 2005 study of 50 of them, researchers found that the normal range of VHS values for cavaliers was from 10.0 to 11.7. In an October 2018 article, a cavalier with a normal-sized heart with a VHS value of 11.9 was reported. In an October 2023 article in which 167 CKCSs with normal-sized hearts were examined, their VHS values ranged from 8.5 to 12.5. These wide ranges of normal-sized VHS values make efforts to diagnose heart enlargement with a single set of x-rays pointless, unless the VHS value is above 12.5.
In the 2016 EPIC Study, its lead investigators included in their arbitrary definition of enlarged hearts in MVD-affected dogs, a maximum VHS value of 10.5 for all dogs of all breeds and mixed breeds. Specifically, the EPIC Study definition of an enlarged heart included: "... radiographic evidence of cardiomegaly (vertebral heart sum (VHS) > 10.5)." This definition was an absurdity for at least three reasons: (1) VHS values of cavaliers with MVD-clear hearts have been reported as high as 11.9, and other breeds have average VHS values for healthy hearts in excess of 10.5; (2) VHS values of healthy hearts are necessarily breed-specific because different breeds have different sizes of hearts and even different sizes of vertebrae and intervertebral disk spaces; and (3) the best use of the VHS method is to compare VHS values of the same dog in periodic x-rays over time.
In a June 2020 article, University of California at Davis veterinary researchers performed fluoroscopic scans of the hearts of 49 dogs during their cardiac cycles to determine what impact the heart's pumping action may have upon x-ray image measurements to determine the dogs' VHS. Fluoroscopy is a series of low dose x-ray images that show internal organs in motion. Fluoroscopy produces poorer quality images than x-rays and therefore is not as accurate for diagnostic purposes. However, since fluoroscopic images can show a series of images of the heart during its cycling, they were useful in this study in assessing changes in the quantitative measurements of the heart during its cycle. In this study, the investigators measured VHS at the point of end-diastolic volume (the maximum amount of blood in the left ventricle just before the heart contracts) and end-systolic volume (the minimum volume of blood in the ventricle at the end of contraction). They report finding that the cardiac cycle had a significant impact on VHS (mean difference: 0.36 ± 0.14 vertebral units between systole and diastole). They concluded that "The cardiac cycle has a significant effect on VHS... . These findings should be taken into consideration during clinical use of these measurements, especially if a patient is being monitored for cardiac changes over time via serial radiographs."
In a November 2024 article, Thai veterinary specialists compared computed tomographic (CT) images of 115 dogs with healthy hearts (including one cavalier) to determine the best position in which to place a dog when obtaining x-rays of its heart. They evaluated and compared positions identified as lateral, dorsal, and adjusted, obtaining VHS values from each. They found that the lateral and lateral long-axis positions had significantly lower VHS values than the dorsal and adjusted positions. They concluded that the dorsal view resulted in a more accurate measurement of the heart size for obtaining VHS values.
RETURN TO TOP
• Heart-to-single-vertebra ratio
In a December 2022 article, University of Napoli, Italy researchers reported having devised a new, objective method to measure the vertebral heart size (VHS) in dogs withc curved spines or other vertebtal alterations. A primary objection to the VHS method of measuring the size of dogs' hearts has been that some dogs, and breeds such as the English bulldog, may have curved spines, making it impossible to measure lineally the length of several vertebrae. These authors have devised a method they call the heart-to-single-vertebra ratio (HSVR), which requires measuring separately the length of each single vertebra from T4 to T8, including the space between each vertebra, in centimeters (cm). (See image below.) The VHS method calls for taking two linear measurements of the heart on an x-ray, the vertical length (LA) and the horizontal length (SA), and then apply those measurments to the same dog's vertebrae, beginning at the thoracic vertebra T4. The sum of LA and SA, as expressed as the number of thoracic vertebrae, is the VHS value for that dog. The HSVR is calculated by dividing the sum of the cardiac long (LA) and short axes (SA) in cm by the length of each vertebral body in cm. This method, in effect, takes the non-linear kinks in a dog's spine out of the VHS equation. The authors report finding that, out of 80 dogs' x-rays in the study, there were "strong correlations between VHS and HSVR" and showed "low bias between the methods". They concluded:
"In the authors' opinion, this new method, which is less time consuming and more objective, could offer a valuable alternative to VHS."
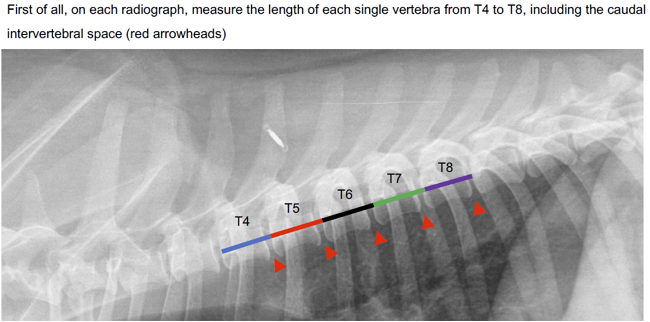
RETURN TO TOP
• VLAS measurements of left atrium
In an October 2018 article, a more highly accurate x-ray measurement of the left atrium (LA) to determine if MVD-affected dogs have LA enlargement, has been introduced. It is called "vertebral left atrial size (VLAS)". The lower broken line of the x-ray in Figure 1 (left below), labeled #, shows the location of the measurement of the LA, and the upper broken line, labeled *, shows how that length is compared to the dog's vertebrae, begining at vertebra T4. Technically, the measurement is described as:
"First, a line was drawn and measured (in arbitrary units) from the center of the most ventral aspect of the carina to the most caudal aspect of the left atrium where it intersected with the dorsal border of the caudal vena cava. For the purpose of this study, the carina was defined as the radiolucent circular or ovoid structure within the trachea that represented the bifurcation of the left and right mainstem bronchi. Similar to the vertebral heart size method, a second line that was equal in length to the first was drawn beginning at the cranial edge of T4 and extending caudally just ventral and parallel to the vertebral canal (Figure 1 below). The VLAS was defined as the length of the second line expressed in vertebral-body units to the nearest 0.1 vertebra."
The researchers compared these x-ray dimensions in 103 dogs divided into four
categories determined by
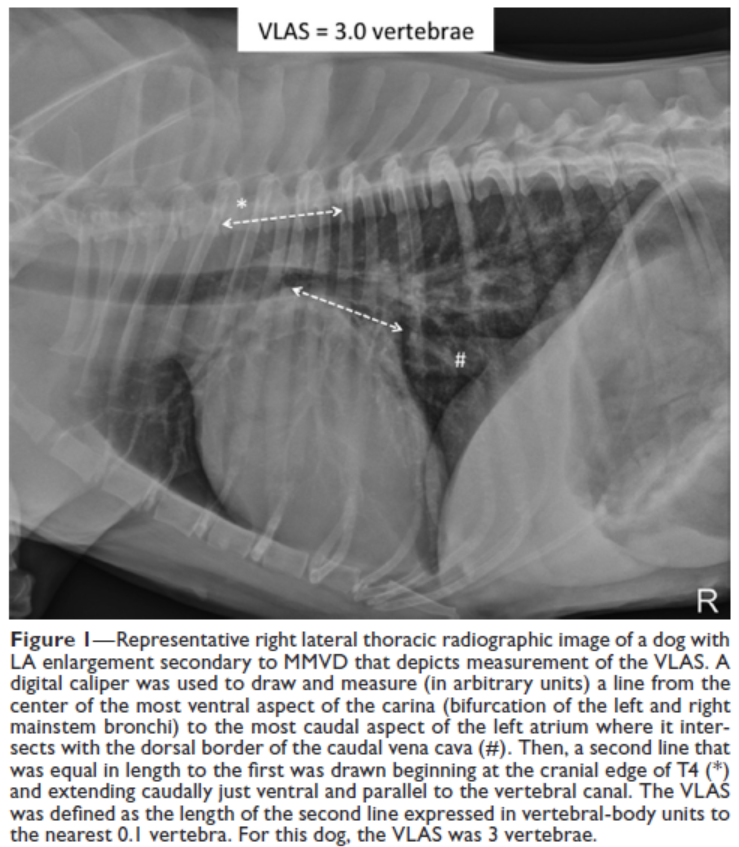 echocardiographic scans: (1) control group of
15 heart-healthy dogs; (2) 40 dogs, including 2 cavalier King Charles
spaniels, in Stage B1 (MVD-affected but without enlargement); (3) 26
dogs (2 CKCSs) in Stage B2 (MVD with enlargement); and (4) 22 dogs (4
CKCSs) in Stages C or D (in congestive heart failure). The investigators compared these x-ray measurements with
echocardiographic measurements of the left atrium of each dog, using two
standard echo images of the LA: (a) left atrium-to-aortic root ratio
acquired from short-axis (LA:AoSx) and (b) long-axis (LA:AoLx). They
conclude:
echocardiographic scans: (1) control group of
15 heart-healthy dogs; (2) 40 dogs, including 2 cavalier King Charles
spaniels, in Stage B1 (MVD-affected but without enlargement); (3) 26
dogs (2 CKCSs) in Stage B2 (MVD with enlargement); and (4) 22 dogs (4
CKCSs) in Stages C or D (in congestive heart failure). The investigators compared these x-ray measurements with
echocardiographic measurements of the left atrium of each dog, using two
standard echo images of the LA: (a) left atrium-to-aortic root ratio
acquired from short-axis (LA:AoSx) and (b) long-axis (LA:AoLx). They
conclude:
"Results of the present study indicated that there was a significant positive correlation between VLAS and both LA:AoSx and LA:AoLx, and VLAS cutoffs of 2.3 to 2.5 vertebrae were associated with an LA:AoSx > 1.6 and LA:AoLx > 2.6 (or both). Thus, a VLAS > 2.3 vertebrae can be used as a radiographic indicator of LA enlargement, and dogs with a VLAS > 2.3 vertebrae likely have hemodynamically important MMVD. ... Results of the present study indicated that VLAS was an accurate predictor of LA enlargement in a large and diverse population of dogs with MMVD of varying severity. There was a moderate positive correlation between VLAS and echocardiographic estimates of LA size by linear measurement methods in both the long (LA:AoLx) and short (LA:AoSx) axes. Results also indicated that VLAS was a readily repeatable measurement, with a high level of agreement among measurements obtained by the same individual on multiple occasions as well as by multiple individuals.
In a June 2020 article, University of California at Davis veterinary researchers performed fluoroscopic scans of the hearts of 49 dogs during their cardiac cycles to determine what impact the heart's pumping action may have upon x-ray image measurements to determine the dogs' VLAS. Fluoroscopy is a series of low dose x-ray images that show internal organs in motion. Fluoroscopy produces poorer quality images than x-rays and therefore is not as accurate for diagnostic purposes. However, since fluoroscopic images can show a series of images of the heart during its cycling, they were useful in this study in assessing changes in the quantitative measurements of the heart during its cycle. In this study, the investigators measured VLAS at the point of end-diastolic volume (the maximum amount of blood in the left ventricle just before the heart contracts) and end-systolic volume (the minimum volume of blood in the ventricle at the end of contraction). They report finding that the cardiac cycle had no significant impact on VLAS. They concluded that "The cardiac cycle ... does not impact VLAS."
In a July 2020 article, a team of Italian veterinary investigators compared measuring the vertebral heart size (VHS) method with the vertebral left atrial size (VLAS) method on x-rays of 80 healthy dogs (including only one cavalier King Charles spaniel and 23 Chihuahuas [29%]) and also to determine VLAS reference intervals for healthy dogs. They used right lateral x-rays and reported finding a positive correlation between VHS and VLAS values and no effect of body weight, sex, or age on VLAS. They concluded that VLAS was a repeatable (meaning, different examiners reached the same measurements) method to quantify left atrial size. The main limitation they acknowledged was that:
"[N]o brachycephalic "screw-tailed" breeds (eg, English Bulldog, French Bulldog, Pug, Boston terrier) were included. These breeds present a typical barrel-shaped thoracic conformation in comparison to other dogs, and a greater occurrence of thoracic vertebral body anomalies that could alter the measurement of vertebral-based methods. The reference interval for VLAS determined in this study may thus not be applicable to brachycephalic dogs."
In a May 2022 article, Korean researchers performed periodic VHS and VLAS examinations of 41 dogs diagnosed with MVD prior to Stage C (heart failure - CHF). They reported finding that changes in the VLAS per month [ΔVLAS/month] "showed high diagnostic accuracy in distinguishing which dogs would develop CHF within 180 days and which would not." They concluded that "VLAS and ΔVLAS/month in dogs with pre-clinical MMVD may be useful to identify dogs at risk of developing CHF within the next 180 days." An increase in VHS value of >0.1 per month over six months or more, in combination with a VLAS value >3.0, is evidence that the patient's heart has enlarged enough to classify it in Stage B2.
In a July 2023 article, three researchers at the Istanbul University studied the hearts of 24 cavaliers -- 6 in Stage A (the control group), 6 in Stage B1, 6 in Stage B2, and 6 in Stage C -- to determine the VLAS (Vertebral Left Atrial Size, using x-rays of the dogs' hearts) and compare those VLAS measurements to echocardiographic measurements of the 2019 ACVIM stages of mitral valve disease (MVD). They report and confirm several now obvious findings about left atrium and overall heart size changes as MVD progresses, such as "VHS [Vertebral Heart Size] and VLAS values were significantly higher in the MMVD group than in the control group." They conclude:
"As a result of this study in Cavalier King Charles spaniel dogs, a cut-off VLAS value >2.25 in dogs with MMVD was found to be a cost-effective, easy-to-use, and reliable method to detect left atrial enlargement and monitor disease. However, the atrial enlargement does not always point in the same direction because of the overall structure of the heart, pulmonary edema, effusion, mass formation, and so on. Considering the limitations of radiographic diagnosis in the presence of additional diseases and the possible inclusion of breed-specific differences, new studies on this topic are needed."
RETURN TO TOP
• Modified-Vertebral Left Atrial Size (M-VLAS)
In a February 2021 article, a team of Australian cardiology investigators devised a modification of the Vertebral Left Atrial Size (VLAS) method of measuring the size of the left atrium from x-rays of dogs diagnosed with mitral valve disease (MVD). As they explain it:
"The original VLAS method aimed to quantify LA [left atrium] size radiographically based on a single-dimensional measurement that roughly represented the transverse diameter of the LA body. Yet, LA functional anatomy and geometry is complex, with the left atrium described as a cylinder with an almost fixed head and distensible walls attached to a piston (ie, mitral annulus). The process of LA remodeling is equally complex and non‐uniform. Recognizing the limitations of only using a single 2D lateral radiograph to quantify the complex LA anatomy, the M-VLAS method aimed to complement the existing VLAS method with an additional aspect of measurement, incorporating the dorsoventral dimension of the LA chamber. The goal was to capture dogs with LA remodeling secondary to MMVD that would have otherwise been missed by the VLAS method given its potential limitations as a single-dimensional measurement."
They examined the x-rays of 64 MVD-diagnosed dogs, including 12 cavalier King Charles spaniels (18.75%). They categorized them according to the ACVIM's 2019 stages of MVD (Stages B1, B2, and C). They then measured their new M-VLAS method of calculating the size of the LA and compared it to the VLAS method as well as the Vertebral Heart Size (VHS) and Radiographic Left Atrial Dimension (RLAD) methods, to determine the most accurate in detecting enlargement of the LA. (See Figure 1.) They concluded that:
"M-VLAS, which is superior to VHS, offers an accurate and repeatable way to radiographically identify LA enlargement in dogs with MMVD [myxomatous mitral valve disease]."
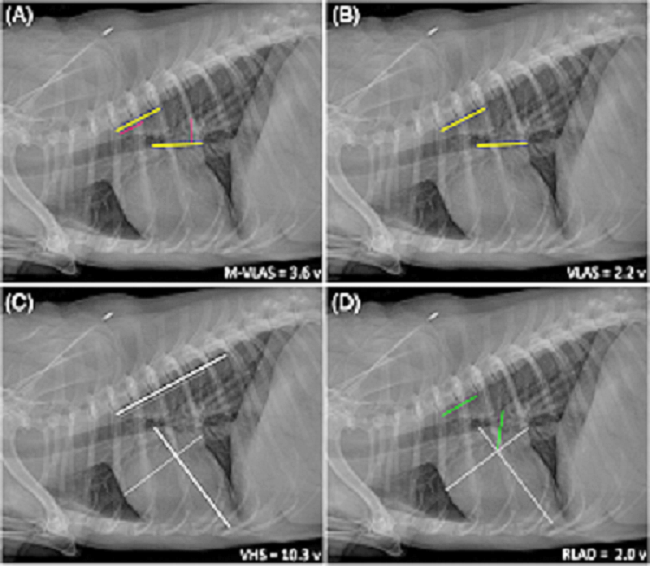
In a February 2023 article, a team of Korean veterinary researchers assessed whether a modified version of the vertebral left atrial size (VLAS) method could be used as an indicator to detect heart enlargement in MVD-affected dogs. They studied the the x-rays of 57 small breed dogs diagnosed with mitral valve disease (MVD). They noted that in some x-rays, the margins of the caudal vena cava (CdVC) could not be seen clearly. To remedy this problem, they measured what could be observed in the x-rays. As they explained it (in technical terms):
"The m-VLAS was measured as the distance from the ventral aspect of the carina to the dorsal aspect of the intersection of the cardiac silhouette and the farthest LA caudal margin, not the CdVC, followed by drawing the same line beginning at the cranial edge of T4."
They concluded that their m-VLAS is a more specific indicator than the VLAS for predicting left side heart enlargement in small breed dogs, and that the m-VLAS can be used as a clinically useful radiographic measurement alternative to or better than the VLAS.
RETURN TO TOP
• Radiographic Left Atrial Dimension (RLAD)
In a
December 2018 article, a team
of European
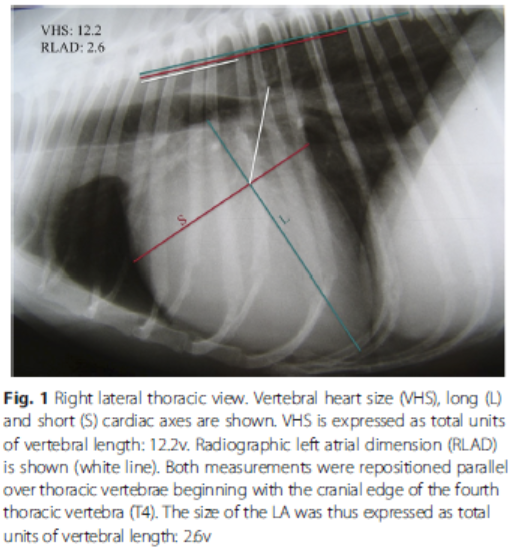 cardiology
researchers report having devised an addendum to the
verterbral heart size (VHS) method of measuring heart size using x-rays
in order to measure just the size of the left atrium. The method, called
Radiographic Left Atrial Dimension (RLAD), extends a diagonal line from
the from the intersection of the long and short VHS axes to the tip of
the left atrium. See Figure 1, rght. They performed these measurements on the
x-rays of 77 dogs, 31 in group 1 of which had no left atrial enlargement
(and included one cavalier King Charles spaniel), and 46 mixed breed
dogs in group 2, all of which reportedly did have enlargement of the
left atrium. They compared the resulting RLAD measurements with
echocardiographic calculations of left atrium size using the ratio of
the left atrium width measurement over the aorta width measurement
(LA/Ao) and found a "strong correlation" between the two resulting
values. They concluded:
cardiology
researchers report having devised an addendum to the
verterbral heart size (VHS) method of measuring heart size using x-rays
in order to measure just the size of the left atrium. The method, called
Radiographic Left Atrial Dimension (RLAD), extends a diagonal line from
the from the intersection of the long and short VHS axes to the tip of
the left atrium. See Figure 1, rght. They performed these measurements on the
x-rays of 77 dogs, 31 in group 1 of which had no left atrial enlargement
(and included one cavalier King Charles spaniel), and 46 mixed breed
dogs in group 2, all of which reportedly did have enlargement of the
left atrium. They compared the resulting RLAD measurements with
echocardiographic calculations of left atrium size using the ratio of
the left atrium width measurement over the aorta width measurement
(LA/Ao) and found a "strong correlation" between the two resulting
values. They concluded:
"The new radiographic measurement named RLAD demonstrated high sensitivity and specificity for detecting LAE [left atrial enlargement] with a strong correlation with LA/Ao ratio. The proposed optimal cut-off value for RLAD to detect LAE is 1.8v. RLAD would provide clinicians with a simple and cost-effective tool for the detection and monitoring of LAE in dogs with MVD and possibly dogs with other cardiac diseases presenting with LAE."
RETURN TO TOP
• Vertebral Heart Area Ratio (VHAR)
The vertebral heart area ratio (VHAR) is two-dimensional, measuring the heart area in relation to the fourth thoracic vertebra (T4) body area (heart area/T4 body area) in dogs. Mathematically, VHAR = heart area/the fourth thoracic vertebra (T4) body area. In a November 2023 article, Korean researchers devised both the VHAR and the vertebral cardiac volume ratio (VCVR), which is CT-calculated cardiac volume divided by the body volume of the fourth thoracic vertebra (T4).
In that November 2023 article, the authors compared VHS measurements and VHAR on x-rays of 125 heart-healthy dogs with CT (computed tomography) images to calculate the VCVR. The boundaries of the heart were measured. The "area" measurement tool of a software program traced the contour of the heart silhouette. The area of the T4 body was measured by drawing along the boundary of its vertebral body.
RETURN TO TOP
• Left Atrial Width (LAwidth)
In a
May 2020 article, University of Wisconsin researchers devised a
specific linear measumrement of the left
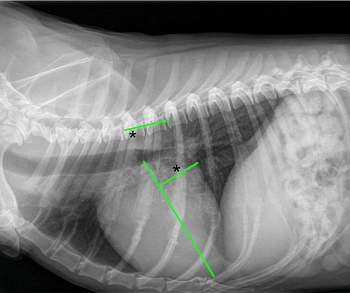 atrium,
called the "left atrium width" (LAwidth). They started with the VHS's
vertical line (from the top to the bottom of the heart -- the
VHSlength), and then extended a straight line perpendicular from the
VHSlength to the edge of the left atrium where it intersects with the
caudal vena cava (the vein carrying blood from the lower part of the
dog's body to the right atrium). (See the green lines diagrammed x-ray
at right.) They compared the length of that perdendicular horizontal
line to vertebral body units (VBUs). (See the green line along the
vertebrae in the x-ray at right.) Specifically, they defined the LAwidth
this way:
atrium,
called the "left atrium width" (LAwidth). They started with the VHS's
vertical line (from the top to the bottom of the heart -- the
VHSlength), and then extended a straight line perpendicular from the
VHSlength to the edge of the left atrium where it intersects with the
caudal vena cava (the vein carrying blood from the lower part of the
dog's body to the right atrium). (See the green lines diagrammed x-ray
at right.) They compared the length of that perdendicular horizontal
line to vertebral body units (VBUs). (See the green line along the
vertebrae in the x-ray at right.) Specifically, they defined the LAwidth
this way:
"The LAwidth was the VBU conversion of the distance of a line drawn perpendicular to the line of the VHSlength and extending caudally to the dorsal aspect of the intersection of the cardiac silhouette and caudal vena cava."
These Wisconsin researchers report that the cut off VBU value of the LAwidth for the dogs in their study was, optimally 2.00 and maximumally 2.25. In this December 2022 article, German researchers explained that:
"The LAwidth was measured as defined by Stepien et al. Again, the long axis was measured as for VHS. The LAwidth line was drawn as short axis at a 90º angle to the long axis from the intersection point of the cardal border of the cardiac silhouette and the dorsal border of the caudal vena cava to the long axis. This LAwidth line was applied to the spine, beginning at the cranial border of T4, and vertebral units were measured as described above."
In this German study, they report that of the four radiographic scores they obtained (the othere were VHS, RLAD, and VLAS), the LAwidth "showed to have the most diagnostic accuracy in differentiation between stages B1 and B2." They concluded that a LAwidth cut off value of 1.8 could be considered for allocating MVD-affected dogs of all breeds in Stage B2 rather than in Stage B1.
• Subjective analysis of x-rays of MVD-affected hearts
A more subjective method of determining heart enlargement from x-rays is
to compare the shape of the left
artium
(LA) and left ventricle (LV) to the known
customary shapes of normal canine hearts, particularly of the same
breed.
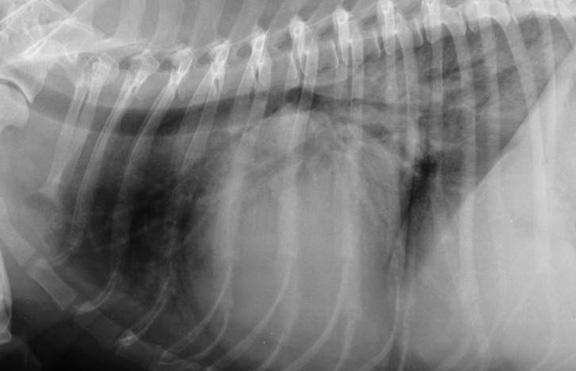 The cardiologists look for a more rounded appearance, bulging,
and other deviations from the typical normally shaped heart, and whether
the heart is impacting the trachea, in
addition to the size of the heart.
The cardiologists look for a more rounded appearance, bulging,
and other deviations from the typical normally shaped heart, and whether
the heart is impacting the trachea, in
addition to the size of the heart.
RETURN TO TOP
• Left Atrium Enlargement (LAE)
Mild left atrial enlargement (LAE) is indicated by a small bump. For moderate LAE, that bump would be clearly enlarged but not huge. For severe LAE, the bump would appear to be huge on the x-rays. Commonly used criteria for LAE include what are called "Roentgen signs" (named after the discoverer of the x-ray). These signs are: (1) elevation of left mainstem bronchus, (2) loss of curvature of the caudal heart, (3) straightening of the caudodorsal margin of the cardiac silhouette, (4) increased height of cardiac silhouette, (5) divergence of mainstem bronchi, (6) double opacity LA, and (7) enlarged (bulging) left atrium.
RETURN TO TOP
• Left Ventricle Enlargement
The increase in size of the LV does not
include a bump. Instead, the enlarged LV is more rounded than a flatter,
normal sized LV. In the x-ray above (Fig. 5), the LA is
outlined in red. In the x-ray at right, the severely enlarged heart is
in the center of the photo, beneath the horizontal spinal column. The LA
is the bulging upper right corner. The LV is the rounded portion on the
right side, beneath the LA.
 To
compare x-rays of the left ventricle (LV) in a better perspective, here
at the left are computer-designed models of a normal sized LV on the
left and an enlarged LV on the right. Note that the healthy LV is
flatter (elliptical), and the enlarged LV is wider and rounded
(globular). (Image from
Ljungvall, et al. 2011.) Dr. John Bonagura has described
the appearance of an enlarged LV as a "squashed mushroom".
To
compare x-rays of the left ventricle (LV) in a better perspective, here
at the left are computer-designed models of a normal sized LV on the
left and an enlarged LV on the right. Note that the healthy LV is
flatter (elliptical), and the enlarged LV is wider and rounded
(globular). (Image from
Ljungvall, et al. 2011.) Dr. John Bonagura has described
the appearance of an enlarged LV as a "squashed mushroom".
RETURN TO TOP
• Quality and standardization of x-rays
A downside of relying upon x-rays to detect heart enlargement is the necessarily subjective analyse of the films. Even experienced board certified cardiologists will argue over whether a single set of x-rays shows left side enlargement or not, and if so, its degree. This usually is due to poor positioning of the dog during the x-rays, which is not uncommon.
Two x-rays of the same dog, even taken at the same session, can vary the appearance of the heart. Variations can be due to (1) the stage of the dog's respiration (inhaling versus exhaling), (2) the stage of the heart's contraction, (3) the centering of the x-ray beam, and (4) the position of the dog on the x-ray table, among a few causes. Ideally, all x-rays should be taken as close to the dog's peak inhalation of its breath as possible. This may be very difficult when the x-rays are taken digitally, because there may be a delay between pushing the button and the actual x-ray beam. Even with perfect timing, the heart size changes with each beat -- systole (contraction) and diastole (relaxation). Standardization of x-rays of MVD-affected dogs is very important to assure that when two sets of x-rays of the same dog are compared, the cardiologist is looking at the same position and timing of the dog's heart.
Maybe a board certified cardiologist can tell from x-rays alone that the LA or LV is enlarged, but as noted above, even they will disagree from time to time, and especially if the positioning of the dog (even flexing the neck can cause misleading shadowing) and quality of the films are less than perfect, or the dog was inhaling in one view and exhaling in another or sedated in one and not in the other. See e.g., Why Is Cardiac Radiology So Difficult? a 2004 article:
"In a recent study two experienced observers examined the radiographs of 57 dogs with common congenital cardiac anomalies without access to any clinical information in order to avoid biasing their interpretations. Under these conditions, the observers reached the correct diagnosis in less than 40% cases. This poor result reflects the difficulty observers had identifying shape changes that can occur in radiographs of dogs with enlarged cardiac chambers. Radiographic signs of specific cardiac chamber enlargement (or pulmonary vascular abnormalities) were recognised by both observers in only 20% instances in which they might be expected."
See also, this November 2001 article, which concluded:
"Even in the hands of experienced clinicians, survey radiography is an inaccurate method for diagnosing canine congenital cardiac anomalies because of the difficulty in recognising the classic radiographic signs, which are not present in every case. ... The results of radiography should be considered as only one part of the clinical assessment and an attempt should be made routinely to reconcile the clinical signs with radiographic findings in order to avoid placing unwarranted emphasis on the perceived size or shape of the cardiac silhouette alone. ... Clinicians should be very cautious about diagnosing congenital cardiac disease based on survey radiographs and it is not recommended that owners are given advice about the potential for treatment or the prognosis for suspected congenital cardiac anomalies on the basis of survey radiographs alone."
In a 2012 study, a team of Spanish researchers, issued a new VHS measurement, called Objective VHS.
RETURN TO TOP
• Baseline and periodic x-rays
Baseline x-rays are a set of
radiographs taken either before or immediately after an MVD murmur is
detected. The baseline x-rays are used to compare to later sets to
determine if the heart as enlarged.
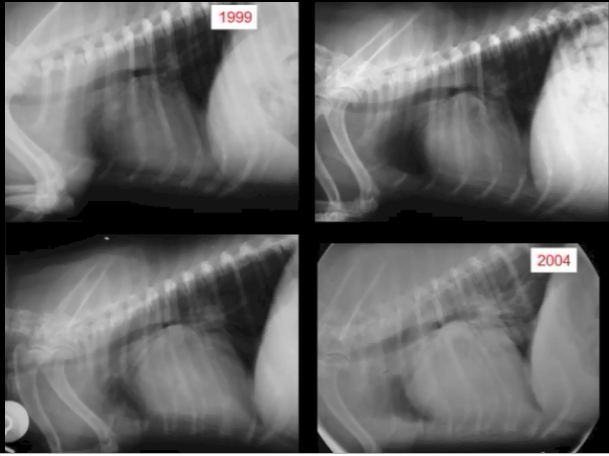 Periodic x-rays of the cavalier's heart, showing the rate of its
enlargement, are viewed by many cardiologists as an effective way to
anticipating the onset of heart failure (HF). (The series of x-rays at
left shows the increase in heart size of one cavalier over 5 years. --
Courtesy of the Royal Veterinary College.)
Periodic x-rays of the cavalier's heart, showing the rate of its
enlargement, are viewed by many cardiologists as an effective way to
anticipating the onset of heart failure (HF). (The series of x-rays at
left shows the increase in heart size of one cavalier over 5 years. --
Courtesy of the Royal Veterinary College.)
The amount of enlargement of the left atrium (mild, moderate, or severe enlargement) usually correlates with the degree of mitral regurgitation (MR) -- mild, moderate, or severe MR.
In a September 2011 study of 94 CKCSs*, an international team of cardiologists concluded that the difference in the vertebral heart scale (VHS) per month was a useful measurement for detecting the onset of HF. A formula used by cardiologists to predict when onset of heart failure will occur, is to determine the change in the vertebral heart scale between two x-rays taken within a year of each other, divided by the number of months between those two x-rays. If that number is equal to or higher than 0.08, then the dog likely will be in heart failure within 12 months. However, this formula is not error-proof and should not be the sole determining factor in predicting or diagnosing the onset of heart failure.
* See follow up letters to editor by Dr. Mark A. Oyama and by the authors regarding this study.
In a January 2013 article, the average VHS measurements of eight other dog breeds -- pug, Pomeranian, Yorkshire terrier, Dachshund, bulldog, Shih Tzu, Lhasa Apso, and Boston terrier -- were studied and listed. See also this November 2017 article which focused specfically on the Dachshund.
RETURN TO TOP
• Diagnosing pulmonary edema and heart failure
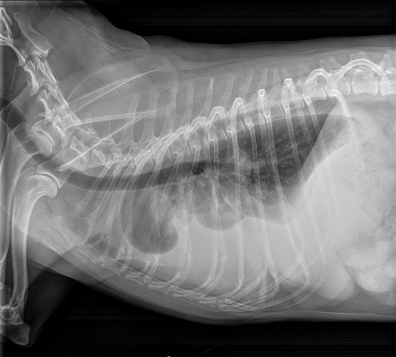 Chest x-rays also are the only diagnostic imaging modality that can
accurately tell if the MVD-affected dog has pulmonary edema (fluid
building up in the lungs) or pleural effusion, meaning has
reached the point of congestive heart failure. Thus, they are
called the "gold standard" for detecting pulmonary edema.
Chest x-rays also are the only diagnostic imaging modality that can
accurately tell if the MVD-affected dog has pulmonary edema (fluid
building up in the lungs) or pleural effusion, meaning has
reached the point of congestive heart failure. Thus, they are
called the "gold standard" for detecting pulmonary edema.
Pulmonary edema is shown on x-rays as an increase in the interstitial density of the lungs. Specifically, the interstitial pattern is described as a diffuse, hazy increase in lung opacity due to the presence of fluids. The more severe the edema, the more dense will be the interstitial markings.
Identifying pulmonary edema on an x-ray alone (without a high respiratory rate) does not confirm a diagnosis of heart failure, because pulmonary edema may have other causes. Therefore, to confirm heart failure as the cause of the edema, there must also be mitral valve regurgitation and enlargement of the left side of the heart.
RETURN TO TOP
• Angiography and Angiocardiography
Angiocardiograms are x-rays made while a radioactive fluid is inected and is circulating through the heart. The fluid is injected through a catheter into the left ventricle, to observe regurgitation through the affected valve. This procedure has been utilized in MVD-affected dogs since the mid-1960s. See this January 1965 article authored by Dr. Buchanan. (See this comparison between a standard x-ray of a dog's heart at leftand an angiogram of the same heart at right, below.)
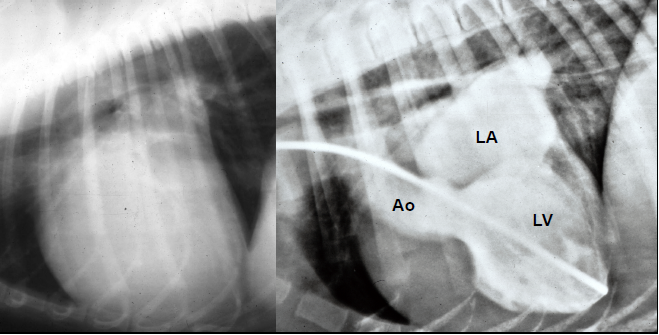
RETURN TO TOP
• Cardiothoracic Ratio (CTR)
Another radiographic method of measuring heart enlargement is called the Cardiothoracic Ratio (CTR), a two-dimensional ratio. The CTR is calculated as the percentage of the cardiac silhouette related to the thoracic area.
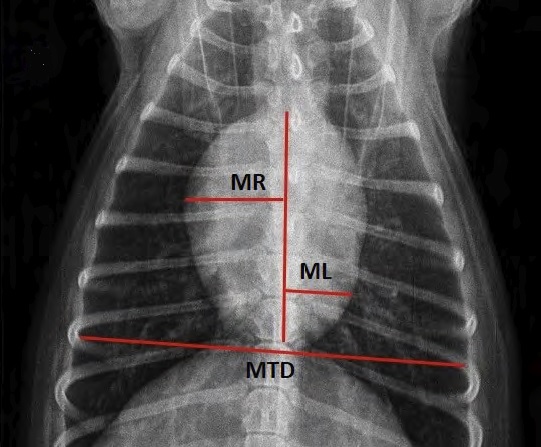 The
CTR was proposed in a
September 2016 article by a team of Brazilian researchers, using a
scheme previously proposed by others for assessing human heart
enlargement. Using the "dorsoventral" x-ray of the dog's heart (see
the photo at right here, from the article), the CTR is calculated
by adding the largest distance from the heart's vertical center line to
the right side of the heart (MR) and the largest distance from that line
to the left side (ML). Then divide that combined amount by the greatest
thoracic diameter (MTD).
The
CTR was proposed in a
September 2016 article by a team of Brazilian researchers, using a
scheme previously proposed by others for assessing human heart
enlargement. Using the "dorsoventral" x-ray of the dog's heart (see
the photo at right here, from the article), the CTR is calculated
by adding the largest distance from the heart's vertical center line to
the right side of the heart (MR) and the largest distance from that line
to the left side (ML). Then divide that combined amount by the greatest
thoracic diameter (MTD).
CTR = MR + ML
MTD
In that article, the Brazilian researchers compared the VHS with the CTR in poodles and concluded:
"Our results demonstrated that VHS and CTR showed strong correlation in their measurements, suggesting that CTR, constantly used in humans, could be considered as a tool to assess the size of the heart silhouette in dogs of the poodle breed."
In a January 2016 article, Romanian researchers reported that the CTR method can discriminate the normal from modified heart size, diagnosed according to the VHS score, in small breed dogs.
In a June 2020 article, University of California at Davis veterinary researchers performed fluoroscopic scans of the hearts of 49 dogs during their cardiac cycles to determine what impact the heart's pumping action may have upon x-ray image measurements to determine the dogs' CTR. Fluoroscopy is a series of low dose x-ray images that show internal organs in motion. Fluoroscopy produces poorer quality images than x-rays and therefore is not as accurate for diagnostic purposes. However, since fluoroscopic images can show a series of images of the heart during its cycling, they were useful in this study in assessing changes in the quantitative measurements of the heart during its cycle. In this study, the investigators measured CTR at the point of end-diastolic volume (the maximum amount of blood in the left ventricle just before the heart contracts) and end-systolic volume (the minimum volume of blood in the ventricle at the end of contraction). They report finding that the cardiac cycle had a significant impact on CTR (mean difference: 2.2 ± 1.2% between systole and diastole). They concluded that "The cardiac cycle has a significant effect on ... CTR ... . These findings should be taken into consideration during clinical use of these measurements, especially if a patient is being monitored for cardiac changes over time via serial radiographs."
RETURN TO TOP
• Manubrium Heart Score (MHS)
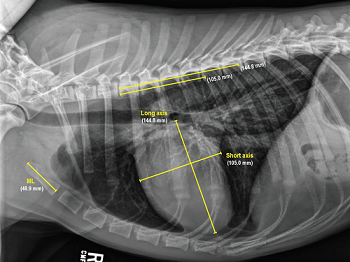 In a
February 2017 article, researchers proposed
assessing cardiac enlargement based upon comparing the length of the
dog's manubrium (the handle-shaped, broad upper part of the sternum,
from which the clavicles and first ribs extend). The manubrium of the
sternum was selected because, the article stated, "it is a relatively
prominent, regularly elongated, bullet-shaped or rectangular bone
segment that is easily identified and can be readily measured on lateral
thoracic radiographic views." The researchers criticized the reliability
of the VHS, stating:
In a
February 2017 article, researchers proposed
assessing cardiac enlargement based upon comparing the length of the
dog's manubrium (the handle-shaped, broad upper part of the sternum,
from which the clavicles and first ribs extend). The manubrium of the
sternum was selected because, the article stated, "it is a relatively
prominent, regularly elongated, bullet-shaped or rectangular bone
segment that is easily identified and can be readily measured on lateral
thoracic radiographic views." The researchers criticized the reliability
of the VHS, stating:
"Additionally, on the basis of the right lateral radiographic findings in this study, the possible variation in the intervertebral disk spaces (eg, intervertebral disk space narrowing) may be another possible factor contributing to the relative weak correlation between the VHS and the corresponding cSAL [cardiac short-axis length] and cLAL [cardiac long-axis length]. However, the canine manubrium is rarely affected by disease, and it is a single prominent, elongated bone segment that can be easily identified on lateral thoracic radiographs. In addition, in the present study, there were strong correlations between the cSAL and cLAL and the corresponding ML. Therefore, we propose that the ML can be used as an appropriate reference value for assessment of the corresponding cSAL and cLAL in dogs."
In an April 2020 article, the same team reported on their study of 184 dogs to evaluate the usefulness of the manubrium heart score (MHS) in distinguishing between dogs with and with cardiac disease. Five cavaliers were included in this MHs study, including one in the healthy control group of small dogs and four in the affected group of small dogs. Their findings included an indication that MHS and VHS were greater in dogs with left-sided cardiac disease, compared to the control group dogs. They concluded that:
"Results indicated that MHSs could be useful, objective radiographic values to help assess dogs for potential heart disease, and we recommend that MHSs be added to the diagnostic tools used by veterinarians when screening for heart disease in dogs."
RETURN TO TOP
• Thoracic Inlet Heart Score (TIHS)
The thoracic inlet heart score (TIHS) is a reference point using the thoracic inlet length to assess tracheal diameter in dogs, with and without MVD. It is intended to overcome the limitations of other methods, such as VHS, VLAS, and RLAD, due to vertebral malformations, conversion to vertebral units, manubrium malformations, and breed variations. See this January 2023 article.
The TIHS is obtained by starting out with the two VHS measurements. The length of the long and short axes of the cardiac silhouette, measured for the VHS, are added together and that sum is divided by the thoracic inlet length (TI). The TI is the distance extending from the cranio-ventral aspect of the first thoracic vertebra to the craniodorsal manubrium at its highest point, the point of the minimum length of the thoracic inlet. The result is the TIHS.
In an August 2023 article, researchers found that among 156 dogs of various breeds (only one cavalier King Charles spaniel), a TIHS value greater than 3.3 would suggest cardiac enlargement in MVD-affected dogs. The intention of the researchers is that if the TIHS value exceeds 3.3, then echocardiography would be recommended for that dog.
RETURN TO TOP
• Dexmedetomidine
Dexmedetomidine often is used to sedate dogs before heart x-rays and echocardiographs. In a January 2016 report, researchers examined the effects of dexmedetomidine on six heart-healthy dogs undergoing chest x-rays and echocardiograms to determine if the sedative caused any changes in the resulting measurements. They found that the x-rays and echos performed after dosing dexmedetomidine resulted in significantly higher measurements of the vertebral heart size and cardiac size, and that moderate to severe mitral regurgitation and mild pulmonary regurgitation occurred in all six dogs. They concluded:
"Findings indicated that dexmedetomidine could cause false-positive diagnoses of valvular regurgitation and cardiomegaly in dogs undergoing thoracic radiography and echocardiography."
View other radiographs of dogs with various stages of MVD here, on the website of the Cardiac Education Group.
RETURN TO TOP
-- respiratory rates (breaths per minute)
 An
ever increasing respiratory rate (called tachypnea), while the dog is asleep, which
approaches or exceeds 30 breaths per minute (bpm), is an indication that the dog is
approaching heart failure. This is because the rise in the bpm is due to
fluid accumulating in the lungs (pulmonary edema).
An
ever increasing respiratory rate (called tachypnea), while the dog is asleep, which
approaches or exceeds 30 breaths per minute (bpm), is an indication that the dog is
approaching heart failure. This is because the rise in the bpm is due to
fluid accumulating in the lungs (pulmonary edema).
Once a dog is diagnosed with MVD and the disease has been progressing, the treating veterinarian may ask the dog's owner to periodically count the number of breaths the dog takes per minute while asleep, and to keep a record of those counts. In an October 2012 study, researching cardiologists found that healthy adult dogs generally have "mean" (average) sleeping respiratory rates (SRR) of less than 30 bpm and rarely exceed that count in the home environment.
While the dog is sound asleep (but not showing signs of dreaming), count the number of breaths the dog takes in 15 seconds. A rise (inhale) and fall (exhale) of the chest count as one full breath or respiration. Then multiply that number by four to get the number of breaths per minute (bpm). If that sleeping respiratory rate (SRR) increases by more than 20 percent over 2 to 3 days, or regularly exceeds 30 breaths per minute, many treating veterinarians would advise the dog's owner to report to them.
Infrequent, extremely rapid breathing rates above 100 bpm are commonly called "panting" (usually over 100 bpm) and should not be counted in this test. The resting respiratory rate (RRR), meaning that the dog is not asleep, is not as accurate as the SRR for determining if the dog has fluid in its lungs.
In a 2011 study which compared the effectiveness of (a) respiratory rate, (b) natriuretic peptide concentration, and (c) echocardiogram, in predicting heart failure, the respiratory rate count was more accurate than both of the other procedures. The researchers stated:
"Only respiratory rate predicted the presence of CHF ... with high accuracy. ... Home monitoring of respiratory rate is simple and very useful in the assessment of CHF in dogs with either DCM or MVD."
In her June 2025 article, Prof. Melanie Hezzell stated:
"Monitoring of sleeping respiratory rate (SRR) is recommended in stage B2, as this allows the early detection of CHF. Patients without CHF or with well-controlled CHF are expected to have an SRR below 30 breaths per minute. We recommend that owners contact a veterinarian for advice if the rate is suddenly and persistently over 40 breaths per minute, or if they notice a trend upwards in the breathing rate over some days."
Every cavalier owner can and should learn this very simple procedure of how to count the breaths of their MVD-affected dogs while they are sleeping. Several cardiologists are recommending that owners become familiar with their MVD-affected cavaliers' normal sleeping breathing rate and effort and keep logs of their sleeping respiratory rates, to establish a baseline rate for each dog, and report when the dogs' rates increase to consistent rates approaching or above 30 to 40 breaths per minute. For example, the University of Pennsylvania's veterinary school advises in a handout available on-line:
"When your dog is at rest, watch their sides rise and fall as they breathe normally. One rise and fall cycle is equal to one breath. Count the number of breaths they take in 15 seconds, then multiply this number by 4 to get total breaths per minute. For example, if you count 8 breaths in 15 seconds, that is equal to 32 (8 x 4) breaths per minute.A normal dog at rest should have a respiratory rate less than 40. If you notice this number increasing consistently, or notice an increase in the effort it takes to breathe, contact your veterinarian."
The vet school at Texas A&M University also has
published a handout
(right) explaining how to keep track of dogs' respiratory rates.
An excellent
YouTube video shows when
![]() and
how every cavalier owner can count the breaths of their MVD-affected dogs
while they are sleeping or at rest.
and
how every cavalier owner can count the breaths of their MVD-affected dogs
while they are sleeping or at rest.
A smart phone app, called Cardalis App, is a useful device for counting respiratory rates. The Android version is available on Google Play, and the Apple version is on iTunes' App Store.
Once the heart failure is controlled by appropriate medication, the respiratory rate may be expected to reduce to a level below 30 breaths per minute. See this January 2016 article.
Bottom Line: Ask your cavalier's cardiologist (you do have one, don't you?) whether and how you should monitor your dog's respiratory rate for congestive heart failure.
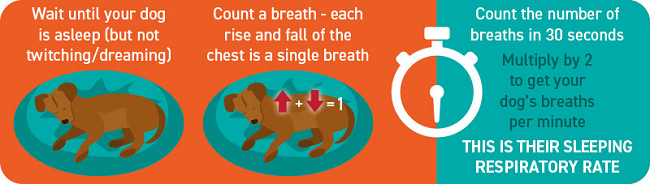
RETURN TO TOP
-- heart beat rates
The "cardiac cycle", or heart beat, consists of the two phases -- contraction and relaxation -- of the heart pumping. When the left ventrical chamber (LV) contracts, the phase is called "systole", and when the LV relaxes, it is called "diastole". The heart rate (HR) is the number of contractions of the heart -- beats -- per minute (bpm).
Changes in the dog's heart rate are tied to changes in cardiac output. Heart rate variability (HRV) reflects variations in the time periods between heart beats. Generally, a low HRV indicates that the dog is under stress, which may be due to exercise, psychological events, or other internal or external events. A higher HRV usually means that the dog has a strong ability to tolerate stress or is strongly recovering from prior accumulated stress. A reduction in HRV in MVD-affected dogs reflects a "sympathovagal imbalance", which has been identified as a risk factor for sudden cardiac death. "Sympathovagal" refers to an interaction between the dog's sympathetic nervous system and its vagus nerve. HRVs reflect the output of the heart as regulated by the sympathetic nervous system. HRV may be determined by using a Holter monitor.
 A
Holter monitor is a non-invasive miniaturized
electrocardiogram device attached to the dog's back, which continuously
digitally records the heart's electrical activity for as many as 48
hours, during the dog's normal activities. The Holter device monitors
heart rhythms and can detect disorders such as syncope. (See a
Holter monitor attached to a cavalier, at right.)
A
Holter monitor is a non-invasive miniaturized
electrocardiogram device attached to the dog's back, which continuously
digitally records the heart's electrical activity for as many as 48
hours, during the dog's normal activities. The Holter device monitors
heart rhythms and can detect disorders such as syncope. (See a
Holter monitor attached to a cavalier, at right.)
In a February 1996 article in which 107 cavaliers were studied, the Swedish cardiologists found that HRV decreased in MVD-affected cavaliers when compared to healthy CKCSs. They also reported that heart rates were significantly increased in cavaliers in CHF.
In a January 2012 study comparing 70 cavaliers with a variety of MVD severity and 20 non-CKCSs in heart failure (CHF) due to MVD, the investigators report that HRV variables decreased in cavaliers with increasing severity of MVD, while the heart rate (HR) increased with increasing severity. Among dogs of all breeds in CHF, HRV variables did not differ.
In a July 2017 study of 34 asymptomatic MVD-affected dogs, compared to 13 healthy dogs, the results showed that MVD dogs had significant higher heart rate (HR) than the control dogs.
In a November 2010 article studying 33 cavalier King Charles spaniels, increased heart rate was been identified as having diagnostic value in identifying cavaliers in congestive heart failure (CHF). However, in a January 2012 study of 256 dogs of several breeds with MVD, heart rate was not among the variables associated with a progression to heart failure.
In a July 2014 report, a Swedish/Finnish/Danish team examined 78 cavaliers with MVD and found that the risk of the onset of congestive heart failure (CHF) increased with a heart rate greater than 130 beats per minute (and a mitral valve murmur grade of 4 to 6). They noted, however, that relying upon increases in heart rates is not always a reliable indication of the onset of CHF.
The Vasovagal Tonus Index (VVTI), a time-domain indicator of heart rate variability obtained from standard electrocardiographic (ECG) recordings, is used to estimate heart rate variability (HRV). In a June 2004 article, the VVTI of four cavaliers in congestive heart failure were significantly lower than those of the eight other CKCSs in ths study. In an October 2017 article, Brazilian researchers report finding from ECG and echocardiographic examinations of 120 MVD-affected dogs, that lower VVTI values were found in dogs in congestive heart failure (CHF -- ACVIM Stage C) compared with MVD-affected dogs not yet in CHF (Stages B1 and B2). They found that values were also lower in dogs with severe cardiac enlargement. They stated:
"When a cut-off value of 6.66 is used, VVTI was able to discriminate MMVD patients in stage C from B1 and B2 dogs with a sensitivity of 70 per cent and a specificity of 77 per cent. MMVD dogs in which VVTI is lower than 6.66 are 30% more likely to develop congestive heart failure (CHF)."
Heart rate variability (HRV) is a measure of the oscillations in the intervals between successive, normal heart beats. Poincare plots are a complicated geometrical method of HRV analysis which is being utilized by some researchers.
RETURN TO TOP
 -- ultrasound (echocardiography)
-- ultrasound (echocardiography)
- Pros and Cons of Echocardiography
- Measurements taken
- Mitral regurgitation
- Leaflet malformation
- Left atrium size and shape
- Left atrial anteroposterior diameter (LADn)
- Left atrial P volume (LAVp)
- Left atrial-to-aortic ratio (LA:Ao)
- Left ventricle shape
- Left ventricle diastolic diameter (LVIDd)
- Ejection fraction
- Transesophageal echocardiography (TEE)
- Simpson's Method
- Mitral E wave and A wave
- Pulmonary-vein-to-pulmonary-artery ratio
- Color flow Doppler
- 3-D echocardiograms
- Tissue Doppler imaging
- The Tei Index
- Left ventricular torsion (LV-Tor)
- Leaflet-annular index (LAI)
- Speckle-tracking echocardiography (STE)
- Tissue motion annular displacement (TMAD)
- Lung ultrasound examination (LUS)
- Measuring pulmonary hypertension
- Sedation
Echocardiography (ultrasound scanning of the heart and connected blood vessels) is a beam of ultra-high frequency sound directed at the heart, and is used to evaluate heart size, function, and valve appearance. The earliest indications of MVD are outwardly invisible and silent and can only be observed by the ultrasound.
T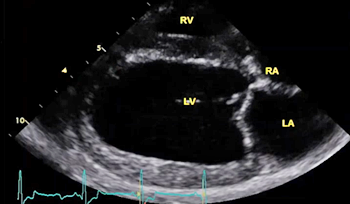 he most common and least invasive type of echocardiography is called
transthoracic echocardiography
(TTE). Echo scans can demonstrate the thickened valve
leaflets and their abnormal movement, such as "tethering", "tenting", and
prolapse (MVP). In one study, 26% of dogs
diagnosed with MVD by echocardiography did not have a murmur.
he most common and least invasive type of echocardiography is called
transthoracic echocardiography
(TTE). Echo scans can demonstrate the thickened valve
leaflets and their abnormal movement, such as "tethering", "tenting", and
prolapse (MVP). In one study, 26% of dogs
diagnosed with MVD by echocardiography did not have a murmur.
The TTE echo scan image at the right shows the four chambers of the dog's heart. "RV" at the top is the right ventricle. "RA" to the upper right is the right atrium. "LV" in the lower middle is the left ventricle. "LA" in the lower right is the left atrium. Between the LV and the LA is the mitral valve.
RETURN TO TOP
• Pros and Cons of Echocardiography
Bottom Line: Ideally, the same sonographer, a cardiologist, should perform all repeat echos of an individual patient, using the same ultrasound device -- for consistency in accuracy and meaningfulness in measurement results.
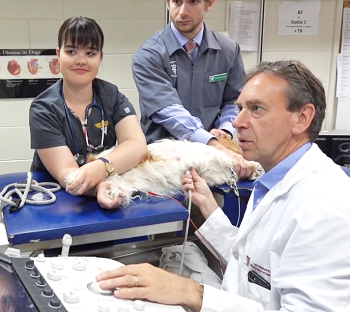 Echocardiography enables the cardiologist to assess the
dimensions and
functioning of the heart chambers. Since MVD affect the size and
function of the chambers, particularly the left ventricle (LV) and left
artial (LA) chambers,
the values determined from echocardiographic scanning are used by the
cardiologist as a tool in diagnosing MVD and its progression and
severity. There currently are one-dimension (M-mode, or
motion-mode or 1-D),
two-dimension (2-D), and three-dimension (3-D) echocardiographic devices
used in veterinary cardiology, with the 3-D measurements being most
accurate and 1-D the least. because the 1-D and 2-D devices require
estimates of chamber volumes, while the 3-D device measures the volumes
without requiring estimations. (Photo at right is of veterinary
cardiologist Dr. Michele Borgarelli viewing on a computer screen an
echocardiographic image of a cavaliers's heart which he is scanning
with the probe [transducer] he holds next to the dog's chest.)
Echocardiography enables the cardiologist to assess the
dimensions and
functioning of the heart chambers. Since MVD affect the size and
function of the chambers, particularly the left ventricle (LV) and left
artial (LA) chambers,
the values determined from echocardiographic scanning are used by the
cardiologist as a tool in diagnosing MVD and its progression and
severity. There currently are one-dimension (M-mode, or
motion-mode or 1-D),
two-dimension (2-D), and three-dimension (3-D) echocardiographic devices
used in veterinary cardiology, with the 3-D measurements being most
accurate and 1-D the least. because the 1-D and 2-D devices require
estimates of chamber volumes, while the 3-D device measures the volumes
without requiring estimations. (Photo at right is of veterinary
cardiologist Dr. Michele Borgarelli viewing on a computer screen an
echocardiographic image of a cavaliers's heart which he is scanning
with the probe [transducer] he holds next to the dog's chest.)
A single echo scan cannot distinguish between a normal-sized left atrium or left ventricle, and a mildly enlarged one. Therefore, the most effective use of echocardiology is periodic serial echo scans, comparing the measurements from an initial echo scan (baseline) with later ones (follow-ups) to determine if size has increased and function has decreased during the interim period between the scans. Ideally, the same ultrasound operator (preferrably a cardiologist) should perform all serial scans, to assure consistency of the axis and plane and stage of the heart's function of the measurements being taken. The heart is constantly changing its shape as it acts as a pump. The filling of the left ventricle is called "diastole"; the ejection of blood from the left ventricle is called "systole". These two stages are broken down to substages -- early and end-diastole and early and end-systole. Capturing the measurements at the exact same substage for each of the serial scans is essential so that all comparisons with the baseline scan are "apples-to-apples".
Echocardiography cannot diagnose MVD heart failure, because it cannot visualize lung congestion -- pulmonary edema -- which is the hallmark of Stage C mitral valve disease, called congestive heart failure (CHF). To detect pulmonary edema, the veterinarian needs to know the dog's breaths-per-minute while it is asleep (the sleeping respiratory rate or SRR), and/or examine chest x-rays or perform a lung ultrasound (LUS), also called a point-of-care ultrasound (POCUS). See Lung Ultrasound, below.
Echo can identify findings that are compatible with MVD heart failure, such as a severely enlarged left atrium or ruptured chordae tendineae. Although a clear mis-nomer, the so-called "gold standard*" test to assess left atrium (LA) size in dogs is the 2-dimensional echocardiography (2DE or 2-D).
* Elevated left atrial pressure (LAP) is the most common sign of MVD in affected dogs. Cardiac catheterization may be called the "platinum" standard because it is the most accurate method of measuring the elevation of LAP. However, catheterization is very invasive and requires anesthesia.
Echocardiography should be considered a complementary technique to radiography (x-ray) and not a substitute. X-rays provide information which echos cannot, particularly regarding overall heart size and shape, while ultrasound enables assessment of the cardiac structures and function.
 Echo
examinations normally are performed on unsedated, restrained dogs, which
may cause increased stress levels and higher heart rates in some dogs.
Also, echocardiography is not a totally reliable means of diagnosing the
condition of the dog's heart. To effectively verify changes in a
dog's heart by echocardiography, such as increases in the sizes of heart
chambers, repeat echos should be performed over a period of months or
even years. This is called serial echocardiograhic variables.
Echo
examinations normally are performed on unsedated, restrained dogs, which
may cause increased stress levels and higher heart rates in some dogs.
Also, echocardiography is not a totally reliable means of diagnosing the
condition of the dog's heart. To effectively verify changes in a
dog's heart by echocardiography, such as increases in the sizes of heart
chambers, repeat echos should be performed over a period of months or
even years. This is called serial echocardiograhic variables.
Ideally, the same sonographer, a cardiologist, should perform any and all repeat echos of an individual patient, and using the same ultrasound devices. Up to now (and the foreseeable future) there exist no uniform guidelines for performing echocardiographic scans on dogs (or cats). There can be wide variability in both the techniques used by the operators, as well as the measurements they obtain. The postions of the patient (lying on its side, legs facing the operator or facing away, or standing), the positions of the transducer (through a cut-out table or from above), how the operator holds the transducer (right), all tend to be user-dependent and with major difficulties in reproducing the same results on the same patient when different operators perform the scans.
Most echo measurements lack precision, because there are many potential sources of error and variability, even under stable conditions with the same sonographer using the same equipment. Cardiologists Drs. John Bonagura and Virginia Luis Fuentes stated in an October 2020 book:
"Many echocardiographic measurements can change by 10% or more because of biologic variability, differences in operator technique, and interobserver factors when measuring images. ... Normal values for dogs and cats are affected not only by species but also significantly by body weight, breed, physiologic variation, plasma volume status, heart rate and rhythm, and measurement errors.
"Most echocardiographic studies are repeatable in experienced hands; however, results can be highly operator and equipment dependent. Foremost, all clinicians should practice within their level of knowledge, training, experience, and expertise, and should not hesitate to send patients to cardiac specialists when referral will serve the best interests of the patient and client." (Emphasis in original.)
In a 1999 study of cavaliers which had no MVD murmur, echocardiographic screenings showed that 82% (54/66) of those dogs aged one to three years had mitral valve prolapse (MVP), and 97% (84/87) of the dogs over age three years had MVP. In a 2015 echocardiographic study of 126 CKCSs in the UK, all aged from 8 to 16 years of age, all dogs had mitral valve thickening and regurgitation; mitral valve prolapse was found in 111/126 (88%), of which 20/111 (18%) were severe; 100% showed echocardiographic evidence of MVD, however a low prevalence of markers of disease severity were found.
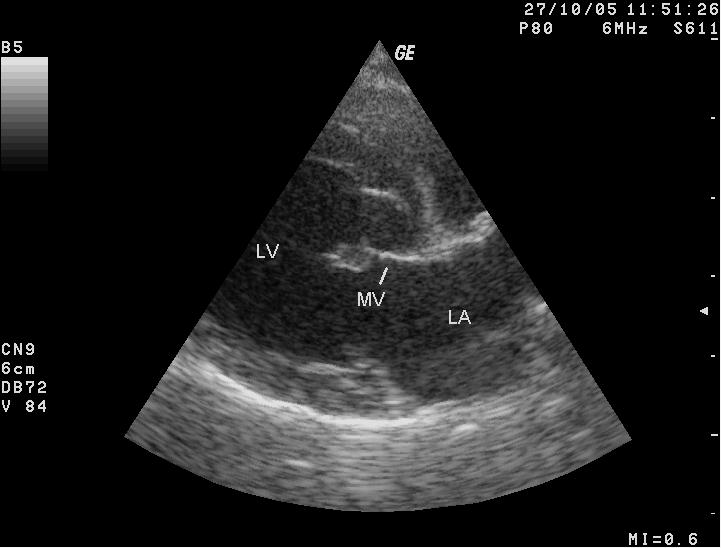
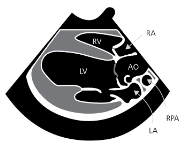
The echo image at left shows a dog's heart with MVD. LA-left atrium; LV-left ventricle; MV-mitral valve, septal leaflet. Note the thickened free end of the mitral valve leaflet at the left of the valve. The diagram at right shows the view more clearly.
RETURN TO TOP
• Measurements Taken
During transthoracic echocardiography (TTE) exams, the operator typically also will take measurements of the heart to determine if it has enlarged and the likely onset of heart failure (HF). Measurements typically include:
(1) the interventricular septal end diastole (IVSd) and end systole (IVSs) in millimeters (mm);
(2) the left ventricular internal diameter end diastole (LVIDd) and end systole (LVIDs) in mm;
(3) the left ventricular end-systolic diameter normalized for body weight (LVIDSn) in mm;
(4) the left ventricular internal diameter end diastole (LVPWd) and end systole (LVPWs) in mm;
(5) the left atrial (LA) in mm, and the aortic root (AO) in mm, to determine that ratio (LA:AO); and
(6) the left ventrical (LV) diastolic volume and the LV systolic volume, from which the operator may calculate the fractional shortening percentage (FS%); and
(7) mitral E wave velocity, which is the peak velocity flow in early diastole.
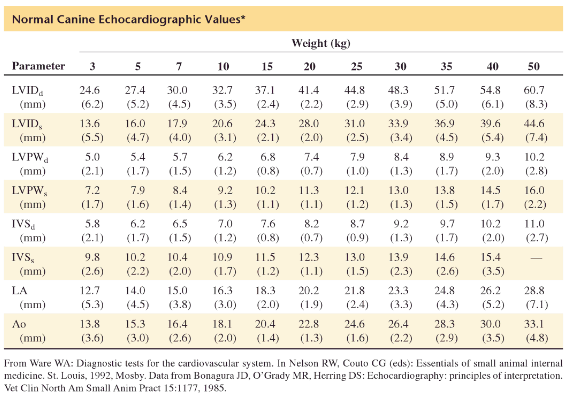
In a November 2014 article, cardiologists comparing mitral valve murmur intensity with the severity of MVD defined cardiac enlargement "as an increase in at least two of the three echocardiographic variables (LA:Ao, wLA and wLVID) above previously established reference intervals."
RETURN TO TOP
• Mitral Regurgitation (MR)
A valuable part of an echocardiographic examination is an assessment for the presence and measurement of mitral regurgitation (MR). The amount of enlargement of the left atrium (mild, moderate, or severe enlargement) usually correlates with the degree of MR -- mild, moderate, or severe MR. MR is the ratio of regurgitant jet area (RJA) to LA area (RJA/LAA) is commonly used as a method to assess the severity of MVD.
Evaluation of MR severity remains imprecise and therefore ideally requires a combination of echocardiographic measurements and parameters. A species-wide set of these parameters include: (a) Regurgitant fraction (>50%, see below); (b) Regurgitant volume (>1.0 mL/kg); (c) E-wave dominant inflow >1.0 m/s); (d) E:A ratio (>2); (e) Triangular regurgitant profile; (f) LVIDdN (>1.9); and (g) LA:Ao (>2.0). Since these parameters are species-wide and do not take into account breed-specific (or individual patient) variations, they necessarily are prone to inaccuracies.
The severity of MR using the regurgitant fraction method can be assessed on color flow Doppler recordings and has been and classified (see this July 2017 article) as:
• No MR = 0%
• Mild MR = 1% to 20%
• Moderate MR = 20% to 50%
• Severe MR = over 50%
• Intermittent MR = no or mild MR in the majority of cardiac systoles but with some MR jets >20%.
In a June 2019 article, the severity of MR was classified thusly:
• Mild MR: <20%
• Moderate MR: 20% to 50%
• Severe MR: >50%
In that July 2017 article, cardiologists who studied the echos of 1,125 cavaliers aged between 1 and 3 years reported that CKCSs in that age group with moderate to severe MR and intermittent MR had significantly increased hazard of cardiac death compared to dogs with no MR. This may seem obvious, but with a 1,000+ group of dogs, it makes it pretty much conclusive for cavaliers. However, this finding was not the case when MR was based on murmur intensity on auscultation. Thus, this emphasizes the importance of assessing MR using echocardiography.
In a September 2017 article, Virginia-Maryland Veterinary School cardiologists Giulio Menciotti and Michele Borgarelli and Utrecht University cardiologist Sandra Muller utilized measurements of the size of the regurgitant gap in the mitral valve -- called the "anatomic regurgitant orifice area" (AROA) -- to determine if that measurement compares to the severity of mitral regurgitation (MR) assessed by an echocardiographic scoring system (MRSS). They devised four classes of the MRSS -- normal, mild, moderate, and severe. They used "real-time three-dimensional transthoracic echocardiography" (RT3DE) to arrive at the AROA dimensions. Eleven cavalier King Charles spaniels were included in the 60 dog, 25 breed, study. They found that The AROA was significantly greater in dogs with a severe MRSS compared with dogs with mild MRSS, and that there was no difference between the AROA of dogs in different ACVIM clinical stages (i.e., Stages B1, B2, C). They concluded that it is feasible to obtain the AROA in dogs with MVD using RT3DE, and that the AROA of dogs with severe MR is greater than the AROA of dogs with mild MR.
Trivial MR: Trivial mitral regurgitation is defined as less than 15% of left atrial area. As many as 50% of dogs with normal hearts and no mitral valve murmurs may have trivial MR. In a July 2024 study of 30 chihuahuas in Japan, 86.7% of the dogs had MR and no murmur. In that study, the timing during the heart cycle for observing MR was noted. Usual MR occurs from early to late systole (called full systolic MR). In that study, late systolic MR, which occurs only during the late systolic phase was observed, suggesting that dogs with only late systolic MR may be predisposed to MVD.
In this July 2025 article, the authors distinguish between diagnosing MVD by means of detecting a murmur using a stethoscope and observing regurgitation which is so quiet that no murmur is heard. They state:
"Although arguably changes in the heart valve attributable to MMVD are present before the onset of a heart murmur, these are only likely to be relevant in a research setting or when early decisions about suitability for breeding must be made."
RETURN TO TOP
• Leaflet Malformation
Echocardiography can depict malformations of the mitral valve leaflets, such as shape, thickening or swelling along the edges of the leaflets, leaflet separation when closed, irregular motion, and loss of function.
RETURN TO TOP
• Left Atrium Size and Shape
During each beat of the heart, the left atrium (LA) constantly changes its shape. Therefore, when measuring the interior dimensions of the LA, most cardiologists select the echo image of the LA at the same point in that cycle, which is from the right parasternal short-axis view at the onset of ventricular diastole -- the first clear frame after aortic valve closure, where all valve cusps are visible, and the aorta has a clover-leaf shape.
 Enlargement
of the heart's left atrium (also called dilation or dilatation, or
cardiomegaly) may occur as MVD progresses, due to the backflow
(regurgitation) of blood through the mitral valve. Therefore, the volume
of the interior of the left atrium (LA) is considered to be one of the
most important echo measurements. Echo calculations of the size using
only linear measurements can be much less accurate than 3-D echo because
LA enlargement can be very asymmetrical, as the x-ray image of the LA
(at right outlined in red)
demonstrates. Nevetheless, most echo measurements of LA size are only
one dimensional (M-mode) or two dimensional (2-D) measurements of the LA
diameter, which can result in a lack of correlation with the actual
volume of the LA.
Enlargement
of the heart's left atrium (also called dilation or dilatation, or
cardiomegaly) may occur as MVD progresses, due to the backflow
(regurgitation) of blood through the mitral valve. Therefore, the volume
of the interior of the left atrium (LA) is considered to be one of the
most important echo measurements. Echo calculations of the size using
only linear measurements can be much less accurate than 3-D echo because
LA enlargement can be very asymmetrical, as the x-ray image of the LA
(at right outlined in red)
demonstrates. Nevetheless, most echo measurements of LA size are only
one dimensional (M-mode) or two dimensional (2-D) measurements of the LA
diameter, which can result in a lack of correlation with the actual
volume of the LA.
RETURN TO TOP
• Left Atrial Anteroposterior Diameter Normalized (LADn)
The left atrial anteroposterior diameter (LADn), normalized to body weight, is a linear measurement used to evaluate the left atrium (LA) size. It is considered to be better at identifying an enlargement of the LA than the LA/Ao ratio (discussed below).
In a May 2024 article by Italian cardiology investigators, they report on their study of the atrial dimension and function at the different stages of mitral valve disease (MVD) as defined by the ACVIM's 2019 MVD Consensus Statement -- Stages A, B1, B2, C, and D. They obtained several echocardiograph measurements in addition to the LA/Ao ratio. These measurements included left atrial anteroposterior diameter normalized for body weight (LADn) and left atrial P volume (LAVp). When comparing dogs in Stage A and Stage B1, they report finding significant atrial enlargement in Stage B1 dogs. Specifically, they found that Stage B1 dogs have increased LADn, suggesting that enlargement of the LA already has begun in Stage B1 cavaliers, even though the ACVIM's 2019 Consensus Statement insists that Stage B1 cavaliers have normal sized LAs when in fact their LAs already have been enlarging. They also find that a notable difference in the functioning of the LA between Stages A and B1, and that the active and passive left atrial emptying fractions are useful in detecting dysfunction of the LA in MVD-affected CKCSs.
RETURN TO TOP
• Left Atrial P Volume (LAVp)
The measurement of the left atrial volume at the onset of the P-waves in known as the left atrial P volume(LAVp).
In a May 2024 article by Italian cardiology investigators, they report on their study of the atrial dimension and function at the different stages of mitral valve disease (MVD) as defined by the ACVIM's 2019 MVD Consensus Statement -- Stages A, B1, B2, C, and D. They obtained several echocardiograph measurements in addition to the LA/Ao ratio. These measurements included left atrial anteroposterior diameter normalized for body weight (LADn) and left atrial P volume (LAVp). When comparing dogs in Stage A and Stage B1, they report finding significant atrial enlargement in Stage B1 dogs. Specifically, they found that Stage B1 dogs have increased LADn, suggesting that enlargement of the LA already has begun in Stage B1 cavaliers, even though the ACVIM's 2019 Consensus Statement insists that Stage B1 cavaliers have normal sized LAs when in fact their LAs already have been enlarging. They also find that a notable difference in the functioning of the LA between Stages A and B1, and that the active and passive left atrial emptying fractions are useful in detecting dysfunction of the LA in MVD-affected CKCSs.
RETURN TO TOP
• Left Atrial-to-Aortic ratio (LA:Ao)
In MVD-affected dogs, enlargement of the left atrial (LA) chamber is usually assessed by the "left atrial-to-aortic ratio" (LA:Ao or LA/Ao). The LA:Ao ratio uses the assumed relatively fixed diameter of the aortic root (Ao) to assess the degree of left atrial volume loading. Unfortunately, this assumption -- that the aortic root dimension will not vary -- is a false one, and the LA/Ao ratio is, in fact, an unreliable guide for comparing left atrial size over time.
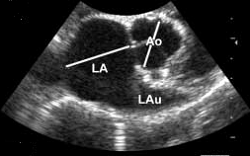 The heart is constantly changing its shape as it acts as a pump. The
filling of the left ventricle is called "diastole"; the ejection of
blood from the left ventricle is called "systole". These two stages are
broken down to substages -- early and end-diastole and early and
end-systole. Capturing the LA and Ao measurements at the exact same
substage for each of the serial scans is essential so that all
comparisons with the baseline scan are "apples-to-apples". Not only is
consistency of the timing of each measurement vital, but so is
consistency of the axis of the measurement -- the short-axis or the
long-axis. Thus, various LA/Ao ratios for the same dog may be only
worthless data unless the timing and axis are identical.
The heart is constantly changing its shape as it acts as a pump. The
filling of the left ventricle is called "diastole"; the ejection of
blood from the left ventricle is called "systole". These two stages are
broken down to substages -- early and end-diastole and early and
end-systole. Capturing the LA and Ao measurements at the exact same
substage for each of the serial scans is essential so that all
comparisons with the baseline scan are "apples-to-apples". Not only is
consistency of the timing of each measurement vital, but so is
consistency of the axis of the measurement -- the short-axis or the
long-axis. Thus, various LA/Ao ratios for the same dog may be only
worthless data unless the timing and axis are identical.
For the purpose of calculating the LA:Ao, the current widely accepted echocardiographic image of the left atrium is obtained from the right parasternal short-axis view at the onset of ventricular diastole -- the first clear frame after aortic valve closure, where all valve cusps are visible, and the aorta has a clover-leaf shape. (See the image of the Ao above.)
While the LA:Ao ratio, if carefully and accurately calculated,
unquestionably can be used to determine the
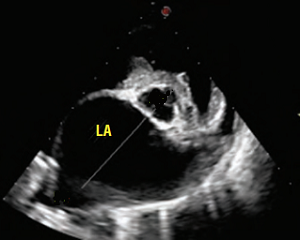 linear width of the LA, that
ratio by itself does not always determine whether the width of the LA means
that the dog's LA is enlarged or not. A comparison of two sets of
LA:Ao measurements -- a baseline set taken previously and a current set
taken months or years later -- will show whether the LA has enlarged in
the interim. But a single measurement of the LA:Ao is static and only
tells what the current dimension is and not whether it is larger than it
has been in the past. However, at a very high ratio, such as 2.0 or
above, enlargement may be safely assumed., especially when that
information is combined with the image of the LA appearing significantly
wide, such as in this image to the left, here.
linear width of the LA, that
ratio by itself does not always determine whether the width of the LA means
that the dog's LA is enlarged or not. A comparison of two sets of
LA:Ao measurements -- a baseline set taken previously and a current set
taken months or years later -- will show whether the LA has enlarged in
the interim. But a single measurement of the LA:Ao is static and only
tells what the current dimension is and not whether it is larger than it
has been in the past. However, at a very high ratio, such as 2.0 or
above, enlargement may be safely assumed., especially when that
information is combined with the image of the LA appearing significantly
wide, such as in this image to the left, here.
Nevertheless, several veterinary cardiologists have arbitrarily equated an LA:Ao ratio of 1.6 as the definitive dividing line between a normal sized heart and an enlarged one on a species-wide basis. This assumption appears to have begun with the 2012 PREDICT Cohort Study in which the authors state, without any statistical support whatsoever: "We defined cardiac remodeling as an LA:Ao of 1.6 based upon 2D echocardiographic examination." That arbitrary and un-scientific tactic has been followed by the 2016 EPIC Study, the 2019 ACVIM Consensus Guidelines, and several others.
Interestingly, the only piece of veterinary literature which any of these articles reference to support their species-wide choice of LA:Ao = 1.6 is a November 2002 article, in which researchers Drs. Jens Haggstrom, Kjerstin Hansson, and Clarence Kvart measured the LA:Ao ratio of 166 CKCSs (that is, only CKCSs and no dogs of any other breed), 56 of which were normal and 110 having varying degrees of severity of mitral regurgitation due to MVD. They found that the LA:Ao ratio for the normal dogs was 1.03 ± 0.09 in 2-D mode, and that the ratio for cavaliers with MVD was 1.61 ± 0.57 in 2-D mode. In that November 2002 study of only cavaliers, the researchers stated:
"In this study, we have deliberately refrained from introducing index ranges for mild, moderate, and severe enlargement, because the grading would be arbitrary and was not included in the study design."
In a March 1995 study by these same researchers, of 79 CKCSs in varying stages of MVD, at least one cavalier with an enlarged left atrium had an LA:Ao as low as 1.31, significantly lower than this new arbitrary species-wide definition of enlargement starting at LA:Ao >1.6.
In a November 2019 article, the cardiologists-researchers reported that 28 (12%) of the 233 dogs in their study, all with normal-sized left atriums, had LA:Ao ratios above 1.6, including Jack Russell terriers, Italian hounds, English setters, collies, Cocker spaniels, boxers, Beagles and mixed breeds. Seven of those healthy dogs had LA/Ao ratios over 1.7.
This LA:Ao method has been discredited for accuracy in diagnosing LA enlargement since a July 2014 article, in which the researchers observed that LA:Ao measurements were far inferior to the bi-plane volumetric assessment, called LA volume indexed to body weight measurements (LA Vol/BW), when comparing the two methods in 82 dogs, including 9 cavaliers. Nevertheless, the LA:Ao measurements continue to be relied upon in many echo examinations of MVD-affected dogs suspected of having enlarged hearts.
In a January 2008 article, an Italian team of researchers, led by Dr. Michele Borgarelli, studied 558 MVD-affected dogs among 36 breeds, with various degrees of mitral valve regurgitation. Among the variables, for cardiac-related death, the only significant variable was LA/Ao >1.7.
In a July 2017 article, cardiologists who studied the echos of 1,125 cavaliers* aged between 1 and 3 years unexpectedly found that CKCSs in that age group with a lower value of LA/Ao significantly increased the hazard of cardiac and all-cause mortality. They noted:
"This was not expected and counterintuitive as it is generally accepted that increasing LA/Ao is a risk factor in MMVD. Again, it should be kept in mind that the dogs are very young and that all LA/Ao values were within a very narrow range and all within reference ranges of normal dogs and thus could represent a finding occurring at random."
*These dogs were privately owned and chosen from a database of CKCS used primarily for breeding purposes.
In a January 2017 article, Bulgarian cardiologist Atanas Pankov compared LA/Ao measurements of 20 MVD-affected dogs with weight idealized aortic size (LA/Aow). He pointed out that weight loss in MVD patients is associated with the progression of the cardiac decompensation and should not be excluded when reporting the progression of the process. He reported that the results from echocardiographic measurements with the weight idealized measured aortic size have a higher reliability in comparison with the LA/Ao ratio method. He concluded that the indexing of M-mode echocardiographic measurements with weight aortic size is more suitable in comparison to the indexing with the linear aortic size, where in the case of this indexing the weight loss with progression of the heart failure is also noted.
In an August 2018 article, Oregon State Univ. researchers discourage the continued reliance upon LA:Ao measurements to determine enlargement. They state:
"Our results also suggest that there are limitations to using a single linear dimension (LA:Ao) to assess LA size. The LA is a complex cardiac structure that can enlarge in multiple planes, so single measurement may not accurately reflect actual LAE [LA enlargement]. This is supported by the stronger agreement of radiographic LAE with LAV [LA volume, calculated by the monoplane modified Simpson's method of discs (MOD)], rather than LA:Ao. Owing to the eccentric manner in which atrial enlargement occurs, large differences in overall LA size may be misrepresented by a small range of LA:Ao values. Enlargement of the LA primarily in the dorsal-ventral orientation may not be apparent in the single plane LA:Ao measurement, while enlargement in multiple directions is incorporated into the LAV value by tracing the LA border. In addition, dividing by the aortic dimension is an attempt to index LA size to a cardiac structure that theoretically does not change much in disease states. However, the size of the aorta may vary throughout the cardiac cycle. While LA:Ao is less time consuming than volumetric measurements, this study and other recent veterinary studies emphasize the limitations of the LA:Ao measurement. Furthermore, the American Society of Echocardiography guidelines recommend that LA size be measured by biplane LAV using the MOD in people. Consequently, we used LAV in addition to conventional LA:Ao measurements to categorize echocardiographic LAE. As there is no established veterinary consensus among cardiologists to delineate degrees of LAE to the authors' knowledge, our results may have differed with the use of different LA:Ao and LAV classification schemes."
In a May 2024 article by Italian cardiology investigators, they report on their study of the atrial dimension and function at the different stages of mitral valve disease (MVD) as defined by the ACVIM's 2019 MVD Consensus Statement -- Stages A, B1, B2, C, and D. They obtained several echocardiograph measurements in addition to the LA/Ao ratio. These measurements included left atrial anteroposterior diameter normalized for body weight (LADn) and left atrial P volume (LAVp). When comparing dogs in Stage A and Stage B1, they report finding significant atrial enlargement in Stage B1 dogs. Specifically, they found that Stage B1 dogs have increased LADn, suggesting that enlargement of the LA already has begun in Stage B1 cavaliers, even though the ACVIM's 2019 Consensus Statement insists that Stage B1 cavaliers have normal sized LAs when in fact their LAs already have been enlarging. They also find that a notable difference in the functioning of the LA between Stages A and B1, and that the active and passive left atrial emptying fractions are useful in detecting dysfunction of the LA in MVD-affected CKCSs.
In a June 2025 article, Korean researchers report inventing "a novel echocardiographic index" called the "Modified-Left-Atrium-to-Aorta Ratio" (M-LA/Ao) in which they take two measurements of the left atrium (LA) instead of just one. Echo scans and x-rays of 136 dogs were studied, none of which were cavaliers. They have added a measurement of the maximum left atrial dimension (LAD) to the formula. Their goal was to evaluate their M-La/Ao ratio with two dimensions of the LA for differentiating between the 2019 ACVIM stages of MVD (Stages B1, B2, and C) and to compare it with other conventional indices. They report that their method "is effective in evaluating LA size in dogs with MMVD, particularly in cases of mild LA enlargement."
RETURN TO TOP
• Left Ventricle Shape
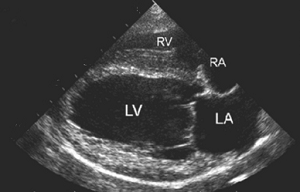 It is possible for some well-experienced echocardiographers to
recognize enlargement of the left ventricle (LV) on the device's screen,
especially if the enlargement is moderate to severe (as opposed to
mild). An enlarged LV will have a more spherical shape than a normal
one, with rounding at its apex and a squashed appearance of the
mushroom-shaped LV lumen at the papillary muscle level. See this
October 2020 article.
It is possible for some well-experienced echocardiographers to
recognize enlargement of the left ventricle (LV) on the device's screen,
especially if the enlargement is moderate to severe (as opposed to
mild). An enlarged LV will have a more spherical shape than a normal
one, with rounding at its apex and a squashed appearance of the
mushroom-shaped LV lumen at the papillary muscle level. See this
October 2020 article.
The heart is constantly changing its shape as it acts as a pump. The filling of the left ventricle is called "diastole"; the ejection of blood from the left ventricle is called "systole". These two stages are broken down to substages -- early and end-diastole and early and end-systole. Capturing the LV measurements at the exact same substage for each of the serial scans is essential so that all comparisons with the baseline scan are "apples-to-apples". Not only is consistency of the timing of each measurement vital, but so is consistency of the axis of the measurement -- the short-axis or the long-axis. Thus, various LV dimensions for the same dog may be only worthless data unless the timing and axis are identical.
RETURN TO TOP
• Left Ventricle Diastolic Diameter (LVIDd)
Measurement of the diameter of the left ventricle (LV) during the heart's end-diastolic stage is obtained during echo scans to estimate the LV volume and determine if LV enlargement has occurred between baseline scans and later ones. This measurement, in centimeters, usually is abbreviated to LVIDd or LVEDd. That calculation then is "normalized" by allometric scaling to the dog's body weight, using an exponent which may vary from 0.294 to 0.315 to 0.33. This result usually is abbreviated to LVIDdN or LVEDdN.
In a May 2004 article, involving 494 normal (meaning no MVD murmurs) dogs of eight breeds plus mixed breeds, including 57 cavaliers, the authors devised an allometric equation to determine an "appropriate" body weight exponent to determine normal-sized left ventricle dimensions on a species-wide basis. They arrived at an exponent of 0.294. The image below demonstrates how they applied that exponent in their calculations. Based upon that 0.294 exponent, they concluded that, "If the result is between 1.27 and 1.85, the value is within the normal prediction interval for this study." Thus, the LVIDdN of 1.85 thereafter was accepted as the maximum for a normal-sized LV in all dogs.
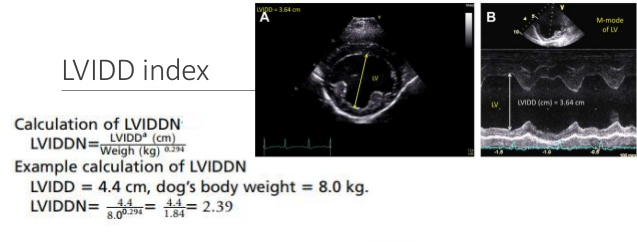
However, in the November 2016 EPIC Study, those authors arbitrarily defined an enlarged LV as having a species-wide LVIDdN of 1.7 or greater, using the same exponent of 0.294. This totally scientifically baseless calculation of 1.7 as the maximum normal-sized LV in all dogs of all breeds also has been adopted by the ACVIM in its 2019 Consensu Statement's re-definition of Stage B2. See this section below for a discussion of the 2019 Stages B1 and B2.
In a June 2022 article, Drs. Mark Rishniw and Donald J. Brown studied the echocardiograms of 1,124 dogs with healthy, normal-sized hearts, including 59 cavaliers. Specifically for the 59 cavaliers in the study, they determined that the upper limits for normal-sized LVs should have a scaling exponent of 0.33 (and thus, not 0.294), and that the range of normal-sized LVIDDNs derived using 0.33 would be from 1.62 to 1.71 with a "mean" (average) of 1.69 for cavaliers.
RETURN TO TOP
• Ejection Fraction (EF%)
The dog's ejection fraction (EF) is an indicator of how efficient the left ventricle is at emptying itself. EF is a percentage measurement based upon the LV's varying volumes. The EF equation is the image of the LV's stroke volume (the amount of blood that is expelled from the heart with each contraction), divided by the end-diastolic volume image. The general rule of thumb is that an EF lower than (<) 40% indicates LV disfunction.
However, in MVD-affected dogs, the EF may be expected to increase or at least remain above 40% due to mitral regurgitation causing an increase in preload of the LV. Since the EF is dependent upon acute changes in left ventricular end-diastolic volume, as the MVD progresses, EF may actually increase before it begins to decline.
•Transesophageal echocardiography (TEE)
 Transesophageal
echocardiography (TEE)* enables imaging of
the heart through the esophagus. Very high resolution two-dimesional
images of the interior anatomy of the heart may be obtained with TEE,
making it useful for monitoring surgical procedures, especially
minimally invasive cardiac operations, in real time, in addition to
diagnoses. In some versions of TEE equipment, 3-dimensional imaging is
an option. An intracardiac echocardiography (ICE) probe for
intravascular imaging also is an option.
Transesophageal
echocardiography (TEE)* enables imaging of
the heart through the esophagus. Very high resolution two-dimesional
images of the interior anatomy of the heart may be obtained with TEE,
making it useful for monitoring surgical procedures, especially
minimally invasive cardiac operations, in real time, in addition to
diagnoses. In some versions of TEE equipment, 3-dimensional imaging is
an option. An intracardiac echocardiography (ICE) probe for
intravascular imaging also is an option.
The dog is placed under general anesthesia in most cases, although some clinicians have had good results with the canine patient being heavily sedated. The ultrasound transducer is attached to a flexible thin endoscopic probe which is inserted through the dog's mouth and down the throat, oropharynx, and esophagus. The position of the transducer can be manipulated to achieve the best direction for detecting the heart. (See the transesophageal echocardiography apparatus at right.) There are risks of injury, considered minimal, to the mouth, tongue, throat, and esophagus, including thermal and hemorraging.
*Also known as transoesophageal echocardiography.
RETURN TO TOP
• Simpson's Method
Another means of measuring the sizes of the left atrium and left ventricle is the "biplane Simpson's method of discs" (SMOD), also known as the "modified Simpson's rule". This is deemed a more accurate measurement method of left atrium and left ventricle volume than the two-dimensional left atrial to aortic ratio (LA:Ao) and left ventricular internal diameter in diastole, normalized for body weight (LVIDDN) because it consists of the summation of measurements of the heart chamber as if it was a stack of elliptical disks.
In an August 2018 article, Oregon State Univ. researchers discourage the continued reliance upon LA:Ao measurements to determine enlargement, preferring the more accurate Simpson;s Method for calculating left atrium volume. They stated:
"Our results also suggest that there are limitations to using a single linear dimension (LA:Ao) to assess LA size. The LA is a complex cardiac structure that can enlarge in multiple planes, so single measurement may not accurately reflect actual LAE [LA enlargement]. This is supported by the stronger agreement of radiographic LAE with LAV [LA volume, calculated by the monoplane modified Simpson's method of discs (MOD)], rather than LA:Ao. Owing to the eccentric manner in which atrial enlargement occurs, large differences in overall LA size may be misrepresented by a small range of LA:Ao values. Enlargement of the LA primarily in the dorsal-ventral orientation may not be apparent in the single plane LA:Ao measurement, while enlargement in multiple directions is incorporated into the LAV value by tracing the LA border. In addition, dividing by the aortic dimension is an attempt to index LA size to a cardiac structure that theoretically does not change much in disease states. However, the size of the aorta may vary throughout the cardiac cycle. While LA:Ao is less time consuming than volumetric measurements, this study and other recent veterinary studies emphasize the limitations of the LA:Ao measurement. Furthermore, the American Society of Echocardiography guidelines recommend that LA size be measured by biplane LAV using the MOD in people. Consequently, we used LAV in addition to conventional LA:Ao measurements to categorize echocardiographic LAE. As there is no established veterinary consensus among cardiologists to delineate degrees of LAE to the authors' knowledge, our results may have differed with the use of different LA:Ao and LAV classification schemes."
In an October 2018 article, the investigators found that the Simpson's method was in agreement with the LA:Ao ratio and demonstrated a good assessment of atrial function. In a February 2020 article, members of that same research team found that The Simpson's method was sensitive to the early detection of left atrial dysfunction and can measure atrial deformation even in asymptomatic dogs, as well as the progressive decline in atrial function as the MVD progresses.
In a March 2021 article, a team of German cardiology investigators applied the Simpson's method a linear method in measuring the left ventricles (LVs) of 1,331 dogs of 128 breeds, including 16 (0.01%) cavalier King Charles spaniels. They produced a single set of reference intervals (the range from the lowest to the highest measurements) for healthy LVs in non-sighthound breeds (see Table 4, below). They conclude:
"Our study provides echocardiographic RIs [reference intervals] for SMOD, independent of breed and size and might enable veterinary cardiologist to use this method for all dogs, using the PLAX [parasternal long axis] or A4C view {apical 4-chamber view] or both."
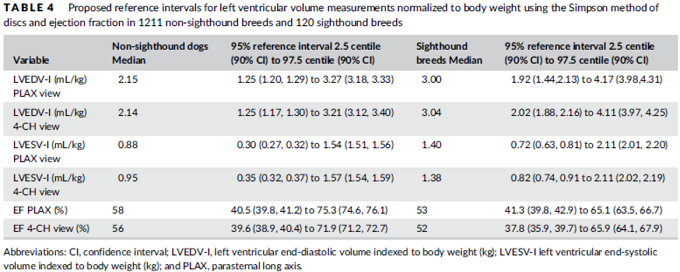
In a June 2021 abstract, University of California-Davis cardiologists used Simpson's method of disks (RF_SMOD) to estimate total stroke volume (TSV) in 81 dogs with MVD and compare it to regurgitant fraction (RF) using M-mode to estimate TSV (RF_M-mode). They also evaluated the effect of pimobendan on RF and the reproducibility of RF. They concluded that RF_SMOD might aid the echocardiographic assessment of MMVD and should be considered for multifactorial approaches to determine disease severity.
RETURN TO TOP
• Mitral E wave and A wave
The peak velocity of the Doppler E (for early) wave in early diastole is considered a measure of mitral regurgitation (MR) severity. E wave usually is measured using pulsed wave Doppler but can be measured using continuous wave Doppler. An E wave velocity (Emax) >1.2 m/sec often is considered a measure of severe MR. See this January 2008 article and this January 2012 article. The E-wave of 100 healthy dogs of a variety of breeds (including 2 cavaliers) has been reported to be 0.58 to 1.17 m/sec in this June 2005 article. (The effect of possible breed-specific differences does not appear to have been considered in these studies.)
The "A wave" reflects the velocity of the flow of blood when the atrium contracts. It is a measure of the combined effect of left atrial contractility and left ventricle compliance. The ratio between the E wave and the A wave is the E/A ratio.This June 2014 article reported that, among 134 healthy cavaliers, the range of E/A was from 1.5 to 2.4. A reduced E/A may indicate a deteriorating left ventricle performance, called "diastolic dysfunction".
RETURN TO TOP
• Pulmonary-vein-to-pulmonary-artery ratio
In a September 2015 article, a team of Belgian researchers performed echocardiographs on 98 dogs -- 61 of which were affected with mitral valve disease (MVD), including 14 cavaliers -- to determine if the pulmonary vein diameter-to-pulmonary artery diameter ratio (PV/PA) can predict congestive heart failure (CHF). They found that the PV/PA index in control dogs equalled approximately 1 and increased with class of heart failure. They concluded:
"The PV/PA is a simple and reproducible echocardiographic variable that increases with class of heart failure and may help discriminate dogs in CHF from asymptomatic dogs with DMVD. Additional studies are required to determine whether PV/PA might provide additional information in the integrated interpretation of Doppler-echocardiographic indices of left ventricular filling pressures and could be used for rapid assessment of CHF in dogs in a critical care setting."
See also this December 2016 article.
In an April 2024 article, investigators compared the progression of MVD using the 2019 ACVIM definitions of the Stages (B1, B2, C, D) to the PV/PA ratio in 80 dogs, of which 65 had been diagnosed with varying degrees of MVD, along with 15 healthy control dogs. None were cavaliers. They measured other echocardiographic indicators of MVD progression, including vertebral heart size, vertebral left atrial size, left-atrium-to-aorta ratio, normalized left ventricular internal diameter, and peak transmitral early diastolic velocity. They report finding these median PV/PA ratios for each category of dogs:
• Control group: 1.03 (0.96-1.24)
• Stage B1: 1.32 (1.11-1.62)
• Stage B2: 1.77 (1.65-2.1)
• Stage C: 2.31 (2.12-2.54)
RETURN TO TOP
• Color Flow Doppler
Color flow Doppler echocardiography provides information regarding mitral valve regurgitation, including the size of the regurgitant jet, its width and spatial orientation, as well as flow convergence into the regurgitant orifice. The color Doppler can evaluate the direction and velocity of blood flow, quantifying blood leakage. It can be used to distinguish MVD from benign murmurs in ambiguous cases. The Doppler may detect leakage before it is audible as a murmur. However, trivial regurgitation of blood through the mitral valve may be present in as many as 50% of normal dogs. In such cases, however, there is no MVP or valve thickening present. Trivial regurgitation ranges up to less than 15% of left atrial area; mild regurgitation ranges from 15% to 50%; modeate to severe regurgitation is greater than 50%. (The color Doppler view below shows red blood at the top, regurgitating from the mitral valve of this CKCS.)
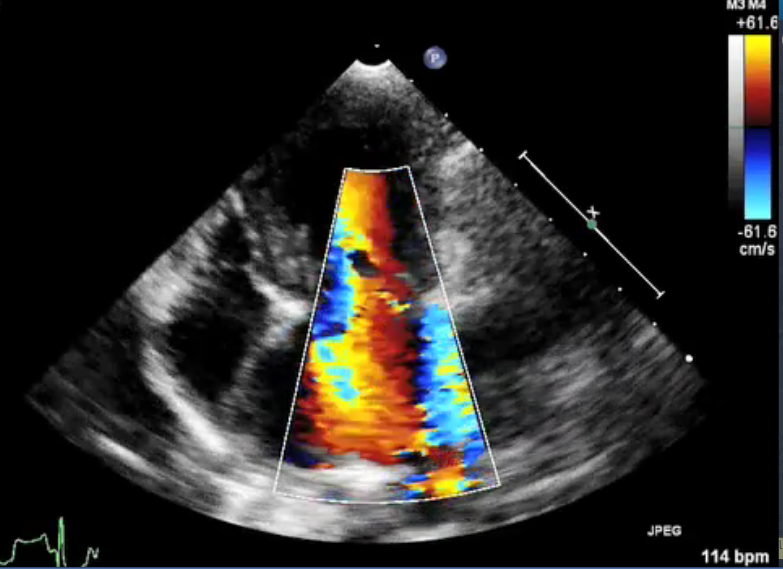
RETURN TO TOP
• 3-D Echocardiograms
 3-D
echos* have enabled cardiologists to more clearly observe the functioning
of the mitral valve, including "tethering" of the valve's leaflets, and
the dimensions of the "tenting" area created during the tethering.
In some instances, 3-D echo measurements of cavaliers' mitral valves
have shown that CKCSs' valves structural features differ from those of other breeds, which
may explain why the onset of MVD in cavaliers is earlier and progresses
more rapidly than in the average canine. In a
September 2016 abstract, an international panel of cardiologists
used 3-D echocardiography to compare the mitral valves of 22 cavalier
King Charles spaniels with 41 other dogs of 18 different breeds. They
measured the dimensions of the mitral valve's annulus (see diagram
at right), tenting (see diagram at left below), leaftet
areas, and several other categories.
3-D
echos* have enabled cardiologists to more clearly observe the functioning
of the mitral valve, including "tethering" of the valve's leaflets, and
the dimensions of the "tenting" area created during the tethering.
In some instances, 3-D echo measurements of cavaliers' mitral valves
have shown that CKCSs' valves structural features differ from those of other breeds, which
may explain why the onset of MVD in cavaliers is earlier and progresses
more rapidly than in the average canine. In a
September 2016 abstract, an international panel of cardiologists
used 3-D echocardiography to compare the mitral valves of 22 cavalier
King Charles spaniels with 41 other dogs of 18 different breeds. They
measured the dimensions of the mitral valve's annulus (see diagram
at right), tenting (see diagram at left below), leaftet
areas, and several other categories.
* 3-D echos are technically referred to as real-time transthoracic three-dimensional echocardiography analysis (RT3DE). There also is transesophageal echocardiogram (TEE), which involves inserting a probe and transducer down the esophagus.
They found that cavaliers had significantly smaller annulus diameter,
annulus height, tenting height, tenting area, normalized tenting volume,
posterior leaflet length, normalized posterior leaflet area, and a
greater annulus sphericity index. They concluded that the mitral valve
of healthy CKCSs was more circular and had less tenting, compared to
other
 breeds.
breeds.
The echocardiograph examination shows the dimensions of the heart chambers, wall thickness and movement, valve movement and lesions, fractional shortening, among other characteristics. The echo screen shows the amount of wall contraction, which enables the operator to determine contractility, preload, and afterload. These factors are used to calculate "fractional shortening" (FS%) which is used as an indication of ventricular performance and of myocardial contractility.
See, also, this March 2017 article, in which the same investigators used 3-D echocardiography analysis on 113 dogs, including 13 cavaliers affected in varying stages of MVD. The 3-D echos enabled the investigators to compare the morphology of the mitral valves (MVs) of healthy dogs (none were CKCSs) and MVD-affected dogs. They report that the study demonstrated that the MVs of MVD-affected dogs differed from those of healthy dogs in several morphological aspects. In particular, the affected dogs had an increased sphericity and a decreased saddle shape of the MV annulus, as well as a decreased tenting height, area and volume. See Figure 1. The study also reportedly demonstrated significant differences in multiple 3-D echo MV measurements between dogs in varying stages (B1, B2, C) of MVD.

In an April 2021 article, Japanese cardiology researchers used three-dimensional transesophageal echocardiography (TEE) to examine 31 MVD-affected dogs, including 9 in Stage B2, 15 in Stage C, and 7 in Stage D. The TEE was performed while the dogs were under anesthesia prior to mitral valve repair surgeries. They found that the annulus height to commissural width ratio of Stage D dogs had significantly lower values than Stage B2 dogs, and that the aortic-mitral angle of Stages C and D dogs were significantly flatter than those in Stage B2. They concluded that the saddle shape of the mitral annulus and aortic-mitral angle were flatter in Stage D than those in the other two stages.
RETURN TO TOP
• Tissue Doppler Imaging
An advanced version of the Doppler ultrasound, called Tissue Doppler Imaging (TDI), has been found to be more sensitive than conventional ultrasound in human medicine. TDI reportedly has been able to detect early myocardial dysfunction in patients with a left ventricular (LV) volume overload induced by mitral regurgitation (MR). TDI also has been tested on dogs and also has been found in a December 2005 study to detect congestive heart failure (CHF). A still more advanced version, known as color Doppler (CD) TDI, was tested in a study published in January 2015 by South Korean researchers. They found that CD TDI was more useful in detecting CHF and that one TDI variable (TDI-derived E/Em sept), which evaluated diastolic function, could be an important predictor of CHF in dogs with MVD.
Contrast echocardiography is a technique for improving echocardiographic resolution in order to assess blood flow. An agitated saline solution provides contrast in the left ventricular (LV) cavity, improving assessment of the left ventricular dimensions, wall motion, and mitral regurgitation.
For cavaliers' hearts, it is recommended that ultrasound scanning be conducted by specialists, preferably board certified veterinary cardiologists.
In an October 2015 report, Drs. Julia Sargent, Virginia Luis Fuentes, and Holger Volk, of the Queen Mother Hospital for Animals at the Royal Veterinary College in the UK, have developed a new echocardiographic scoring system to grade the severity of mitral regurgitation in chronic mitral valve disease, based upon a number of different measurements that they believe can offer more reliable information on the severity of MVD. They are testing their new scoring system by comparing it to cardiac magnetic resonance imaging (cMRI), which is considered the most reliable test for quantifying valve disease in humans. They have determined that the echocardiographic measures that correlated most closely with cardiac magnetic resonance imaging-derived mitral regurgitant fraction were vena contracta/aortic diameter and E-wave velocity.
RETURN TO TOP
• The Tei Index
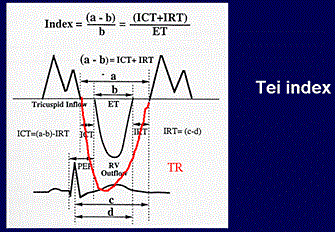 In a
January 2016 article, a team of Hokkaido University, Japan,
veterinary researchers calculated the "right ventricular
Tei-index" (RVTX)* of 30 dogs diagnosed with MVD by echocardiograph. The
dogs were included in Group A (19 dogs) if they survived a year from
their first echo, and in Group B (11 dogs) if they suffered
cardiac-related death within that year. A lack of tricuspid
regurgitation (TR) velocity in 2 of the 11 dogs in Group B disqualified
them. All of the remaining 9 Group B dogs
(which died during the first year) had increased RVTX (>0.61). Three of
the dogs with lower RVTX (<0.61) died before the study ended but
after the first year, and the 18
other dogs with lower RVTX were alive when the study ended.
In a
January 2016 article, a team of Hokkaido University, Japan,
veterinary researchers calculated the "right ventricular
Tei-index" (RVTX)* of 30 dogs diagnosed with MVD by echocardiograph. The
dogs were included in Group A (19 dogs) if they survived a year from
their first echo, and in Group B (11 dogs) if they suffered
cardiac-related death within that year. A lack of tricuspid
regurgitation (TR) velocity in 2 of the 11 dogs in Group B disqualified
them. All of the remaining 9 Group B dogs
(which died during the first year) had increased RVTX (>0.61). Three of
the dogs with lower RVTX (<0.61) died before the study ended but
after the first year, and the 18
other dogs with lower RVTX were alive when the study ended.
Notwithstanding the small number of dogs involved, the researchers concluded:
"The results of the present study indicate that RVTX is strongly correlated with early death in dogs with MMVD. Although several echocardiographic variables were significantly different between the two groups, we found that RVTX, a variable that corresponds to the RV function, was the most significant independent predictor of mortality. This study demonstrates that RV function analysis may be the most reliable prognostic indicator for dogs with MMVD."
*
 In 1995, Dr.
Chuwa Tei (right) devised an index of myocardial performance (the Tei index)
that evaluates the left ventrical (LV) systolic and diastolic function
in combination. The Tei index reportedly is a reliable method for the
evaluation of LV systolic and diastolic performance, with advantages
over previous indexes and prognostic value in many kinds of heart
disease. Calculation of the Tei index is a number calculated from the
ratio of time intervals (a-b/b) determined by pulsed Doppler
echocardiography. See
this December 1995 article,
this November 1996 article, and
this January 2005 article.
In 1995, Dr.
Chuwa Tei (right) devised an index of myocardial performance (the Tei index)
that evaluates the left ventrical (LV) systolic and diastolic function
in combination. The Tei index reportedly is a reliable method for the
evaluation of LV systolic and diastolic performance, with advantages
over previous indexes and prognostic value in many kinds of heart
disease. Calculation of the Tei index is a number calculated from the
ratio of time intervals (a-b/b) determined by pulsed Doppler
echocardiography. See
this December 1995 article,
this November 1996 article, and
this January 2005 article.
RETURN TO TOP
• Left ventricular torsion (LV-Tor)
Another
function of the heart which is observable via echocardiograph
 is
its contraction, measured by left ventricular torsion (LV-Tor). In human MVD, cardiac surgeons
have been focusing on the relative amounts of LV-Tor in patients slated for heart surgery. When the heart contracts,
it essentially is a "wringing" or "twisting" motion by which the top
half of the heart rotates counterclockwise and the bottom half rotates
clockwise. (See diagram at right.) The net difference of the
degrees of rotation of the two halves of the heart is referred to as
"torsion". This activity is observable by echocardiograph. Normal human
hearts show a net torsion of approximately 15º, and as the ejection
fraction decreases and the heart enlarges, torsion also decreases.
Therefore, a reduction in torsion may be a marker of enlargement of the
left ventricle. See this
June 2016 article.
is
its contraction, measured by left ventricular torsion (LV-Tor). In human MVD, cardiac surgeons
have been focusing on the relative amounts of LV-Tor in patients slated for heart surgery. When the heart contracts,
it essentially is a "wringing" or "twisting" motion by which the top
half of the heart rotates counterclockwise and the bottom half rotates
clockwise. (See diagram at right.) The net difference of the
degrees of rotation of the two halves of the heart is referred to as
"torsion". This activity is observable by echocardiograph. Normal human
hearts show a net torsion of approximately 15º, and as the ejection
fraction decreases and the heart enlarges, torsion also decreases.
Therefore, a reduction in torsion may be a marker of enlargement of the
left ventricle. See this
June 2016 article.
RETURN TO TOP
• Leaflet-annulus index (LAI)
In an August 2022 article, Japanese reseachers used echocardiography to measure the mitral valve leaflet-annulus index (LAI) of 83 dogs, including 9 (10.8%) cavaliers, all diagnosed with MVD. The LAI is a complicated calculation which involves taking various measurements of the mitral valve leaflets. It is the ratio calculated with the lengths of the anterior mitral leaflet (AML) and the posterior mitral leaflet (PML) and anteroposterior length (APL) using 2D transesophageal echocardiography (TEE). It is intended to represent the quantity and severity of mitral regurgitation (MR), as it is affected by annular dilation.
The LAI of each dog was compared to other echo measurements, including the left ventricular end-diastolic internal diameter normalized to body weight (LVIDdN), the left atrium to aorta ratio (LA/Ao), and the grade of MR. They report finding that the LAI correlated significantly with both the LVIDdN and LA/Ao. They also observed that the chronic volume overload from MR caused the left ventricle to expand, which in turn caused the mitral annulus to expand, resulting in increased mitral regurgitation.
RETURN TO TOP
• Speckle-tracking echocardiography (STE)
Two-dimensional speckle-tracking
echocardiography (STE) is a novel, angle-independent imaging method that
allows assessment of strain and strain rate in heart chambers, using the
tracking of acoustic speckle patterns.
![]() It is based upon software which
tracks speckles (natural acoustic markers in the myocardium) during the
cardiac cycle. In a
February 2017 article, a team of veterinary researchers at Hokkaido
University in Japan used STE to determine the variations in the extent
of strain in the left atrium in the differing MVD stages. "Strain"
represents deformation of a myocardial chamber over time. It is based
upon the formation of "speckles" -- a series of ultrasonographic
components attributable to reflection, scattering, and interference
between tissue and ultrasound beams -- that can be tracked over time in
2-D echocardiograms. The researchers found that there were no significant differences in parameters of
LA strain between the Stages B1 and B2 groups. However, LA longitudinal
strain during atrial contraction and during ventricular systole were
significantly lower in Stages C and D than in Stages B1 and B2. They
concluded:
It is based upon software which
tracks speckles (natural acoustic markers in the myocardium) during the
cardiac cycle. In a
February 2017 article, a team of veterinary researchers at Hokkaido
University in Japan used STE to determine the variations in the extent
of strain in the left atrium in the differing MVD stages. "Strain"
represents deformation of a myocardial chamber over time. It is based
upon the formation of "speckles" -- a series of ultrasonographic
components attributable to reflection, scattering, and interference
between tissue and ultrasound beams -- that can be tracked over time in
2-D echocardiograms. The researchers found that there were no significant differences in parameters of
LA strain between the Stages B1 and B2 groups. However, LA longitudinal
strain during atrial contraction and during ventricular systole were
significantly lower in Stages C and D than in Stages B1 and B2. They
concluded:
"In conclusion, the results of the present study indicate that LA longitudinal strain was significantly lower in dogs in the advanced stages [Stages C and D] of MMVD. LA longitudinal strain during atrial contraction was the best predictor of the presence or history of CHF. LA strain assessed on conventional echocardiography and radiography could add meaningful information to confirm the presence of CHF in the clinical setting. Further studies are needed to determine the clinical implications of these findings for treatment decisions and/or prognosis determination."
In an April 2017 study of 150 dogs in various stages of MVD, including ten cavaliers, Italian investigators concluded that, "Left atrial STE analysis provides useful information on atrial function in the dog, highlighting a progressive decline in atrial function with worsening of MMVD."
In a February 2018 article, Italian and USA researchers examined 96 MVD-affected dogs (7 were cavaliers) using STE to measure left atrial (LA) longitudinal strain and other variables between Stages B1, B2, and C. They report finding that no STE variables differed between Stage B1 and B2 dogs but were lower in Stage C dogs. They concluded that MVD-affected dogs in CHF appear to have lower LA longitudinal strain and strain rate variables compared with dogs in Stage B.
A review of research into STE in detection of left ventricular dysfunction in dogs is this August 2021 article.
RETURN TO TOP
• Tissue motion annular displacement (TMAD)
Tissue motion annular displacement of the mitral valve (TMAD) is a refinement of speckle-tracking echocardiography (STE) which measures the mitral valve's annular motion, taking the length of the left atrium (LA) and the left ventricle (LV) into account. It can provide an index of LA and LV function. Studies are being conducted using TMAD to distinguish between MVD-affected dogs in the various stages of severity (B1, B2, C, and D) to complement the more standard methods -- x-rays and more traditional echocardiographic examinations.
RETURN TO TOP
• Lung ultrasound examination (LUS)
Lung ultrasound
(LUS) has higher sensitivity than lung auscultation and
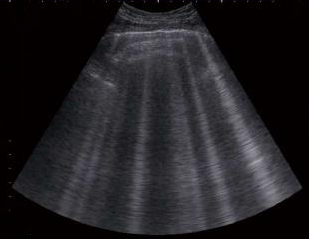 chest
x-rays for many respiratory conditions, such as fluid in the dog's lungs. In a
March 2017 article, a team of Italian researchers used LUS to examine the conditions
of dogs in different stages of mitral valve disease (MVD), to
distinguish lung patterns leading to congestive heart failure (CHF).
They report detecting "B-lines" (also called ultrasound lung rockets)
representing interstitial edema. (See image of LUS B-lines at left.)
The number and distribution of these
B-lines correspond to the presence of extravascular fluid in the lungs
and indicate the severity of pulmonary edema (PE) and stage of its progression. Dogs in
Stage B1 of MVD had absent or rare B-lines in 14 of 15 cases (93.3%);
dogs in Stage B2 had absent or rare B-lines in 16 of 18 cases (88.9%);
all dogs in Stage C without radiographic signs of PE, had absent or rare
B-lines; dogs in Stage C with radiographic signs of PE, had numerous or
confluent B-lines in 18 of 20 cases (90%). They concluded:
chest
x-rays for many respiratory conditions, such as fluid in the dog's lungs. In a
March 2017 article, a team of Italian researchers used LUS to examine the conditions
of dogs in different stages of mitral valve disease (MVD), to
distinguish lung patterns leading to congestive heart failure (CHF).
They report detecting "B-lines" (also called ultrasound lung rockets)
representing interstitial edema. (See image of LUS B-lines at left.)
The number and distribution of these
B-lines correspond to the presence of extravascular fluid in the lungs
and indicate the severity of pulmonary edema (PE) and stage of its progression. Dogs in
Stage B1 of MVD had absent or rare B-lines in 14 of 15 cases (93.3%);
dogs in Stage B2 had absent or rare B-lines in 16 of 18 cases (88.9%);
all dogs in Stage C without radiographic signs of PE, had absent or rare
B-lines; dogs in Stage C with radiographic signs of PE, had numerous or
confluent B-lines in 18 of 20 cases (90%). They concluded:
"Our findings indicate that LUS has good diagnostic accuracy in identifying cardiogenic PE and might be useful in the staging of dogs with CVHD [chronic valvular heart disease]. Lung ultrasound examination isa new, quick, and noninvasive diagnostic tool for the cardiologist, radiologist, or intensive care specialist. It should be considered as complementary to thoracic radiography, and particularly useful when radiographic findings are unclear or in severely dyspneic dogs. In the future, it would be interesting to evaluate the utility of LUS in the chronic management and serial monitoring of dogs with CVHD under treatment."
In a November 2023 article, Italian researchers explored the Lung Ultrasound Score (LUSS), a measurement method that calculates the percentage of the entire pleural surface of the lungs, occupied by the main pattern detected by ultrasound. They report finding "excellent intra- and inter-rater reliability for LUS scoring and pattern identification".
LUS is also referred to as Point-Of-Care Ultrasound (POCUS).
RETURN TO TOP
• Measuring pulmonary hypertension
Pulmonary hypertension -- abnormally high pressures in the pulmonary blood vessels -- is a typical complication of MVD-affected dogs. It normally is measured in humans by cardiac catherization, a complicated invasive procedure which involves inserting a catheter in the right side of the heart. Because of such complications, it rarely is performed in veterinary practices.
Drs. Michele Borgarelli and Jonathan Abbott of the Virginia Tech University's Virginia-Maryland College of Veterinary Medicine are conducting a study comparing pulmonary hypertension by the catheter method with echocardiography. See this article for more information in this on-going study.
RETURN TO TOP
• Sedation
Sedation sometimes is necessary to calm uncooperative dogs during echocardiolgraphic examinations, and in some jurisdictions, sedation is required for all x-rays and echo exams. Intra-muscular injections of dexmedetomidine are commonly used for this purpose. However, side effects of this alpha2-adrenoceptor agonist have been found to decrease heart rate and cadiac output and have other consequences limiting the drug's usefulness for MVE-affected dogs. In a January 2016 report, researchers examined the effects of dexmedetomidine on six heart-healthy dogs undergoing chest x-rays and echocardiograms to determine if the sedative caused any changes in the resulting measurements. They found that the x-rays and echos performed after dosing dexmedetomidine resulted in significantly higher measurements of the vertebral heart size and cardiac size, and that moderate to severe mitral regurgitation and mild pulmonary regurgitation occurred in all six dogs. They concluded:
"Findings indicated that dexmedetomidine could cause false-positive diagnoses of valvular regurgitation and cardiomegaly in dogs undergoing thoracic radiography and echocardiography."
In an August 2024 article, Finnish researchers studied the impact of intravenous (IV) medetomidine and vatinoxan to sedate 12 MVD-affected dogs in Stage B1 (murmur but no enlargement) during echocardiographic evaluations. of dogs with stage B1 mitral valve disease. Four of the dogs (25%) were cavaliers. They report finding "Intravenous medetomidine and vatinoxan caused only mild hemodynamic changes and could be considered safe and useful for sedating dogs with stage B1 MMVD." They cautioned, however, that effects on some systolic and diastolic variables were notable and should be taken into account.
RETURN TO TOP
-- cardiac magnetic resonance imaging (cMRI)
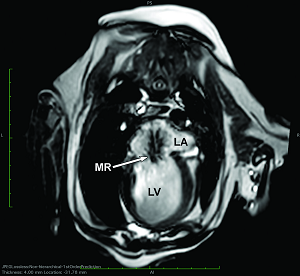 Cardiac magnetic resonance imaging (cMRI) is reported to be an
accurate technique for quantifying ventricular function, left
ventricular (LV) volume and function (including end-diastolic volume,
end-systolic volume, stroke volume, and ejection fraction), as well as
assessing mitral
valve regurgitation and the size of the mitral regurgitant orifice in both humans and dogs. Indeed, cMRI has been
described as a "gold standard" for those measurements. (At right,
see cMRI image of the left-side chambers of a CKCS's heart, showing
mitral regurgitation [MR] between the enlarged atrium [LA] and enlarged
ventricle [LV].)
Cardiac magnetic resonance imaging (cMRI) is reported to be an
accurate technique for quantifying ventricular function, left
ventricular (LV) volume and function (including end-diastolic volume,
end-systolic volume, stroke volume, and ejection fraction), as well as
assessing mitral
valve regurgitation and the size of the mitral regurgitant orifice in both humans and dogs. Indeed, cMRI has been
described as a "gold standard" for those measurements. (At right,
see cMRI image of the left-side chambers of a CKCS's heart, showing
mitral regurgitation [MR] between the enlarged atrium [LA] and enlarged
ventricle [LV].)
Because of the inconvenience and expense of MRI scanning, and the necessity that the dog be anesthetized, in is seldom used for clinical diagnoses. However, it has been used to verify the accuracy of echocardiographic diagnosing.
In a November 2013 article, a team of Korean researchers confirmed the accuracy of contrast echocardiography (CE) compared with unenhanced echocardiography (UE) in measuring LV function. In an October 2015 article, which included cMRIs of cavaliers, UK researchers determined that the echocardiographic measures that correlated most closely with cMRI mitral regurgitant fraction were vena contracta/aortic diameter and E-wave velocity.
In an August 2022 article, Ohio State cardiology researchers examined 4 cavaliers diagnosed with MVD and left chamber heart enlargement, and 2 other MVD-affected dogs, along with 6 clear-heart control dogs, to determine whether cMRI could detect detect myocardial ischemia and fibrosis and also whether circulating biomarker concentrations (cardiac troponin I concentrations and galectin-3 concentrations) could be used as predictors of myocardial changes identified on cMRI. They found some results surprising: While MVD-affected dogs had significantly higher cardiac troponin I concentrations than control dogs, the galectin-3 concentrations did not differ between groups. They also detected myocardial fibrosis was detected in dogs in both groups, and found that no dogs had obvious myocardial perfusion deficits. They attributed the lack of significant differences in myocardial fibrosis between healthy dogs and those with MVD as a possible explanation as to why no veterinary clinical trial has thus far demonstrated a significant survival benefit when dogs with preclinical MVD have been treated with angiotensin-converting enzyme (ACE) inhibitors. They observed that:
"[D]ogs with advanced MMVD may not develop focal areas of myocardial fibrosis significantly more often than what may occur during the normal aging process."
RETURN TO TOP
-- computed tomography (CT)
Computed tomography (CT) is an imaging method using digital geometry processing to generate a three-dimensional image of the inside of an object from a large series of two-dimensional x-ray images taken around a single axis of rotation. Multi-detector row computed tomography (MDCT). with its use of a 64-slice scanner, allows 3-D reconstruction of images and enables the cardologist to make measurements in any desired plane. In studies reported in March 2012, July 2013, and November 2016, MDCT has been found to be an accurate technique for assessing LV volume and function variables, comparable to MRI measurements.
In a November 2023 article, Korean researchers devised the vertebral cardiac volume ratio (VCVR) which is CT-calculated cardiac volume divided by the body volume of the fourth thoracic vertebra (T4).
In a July 2025 article, researchers compared the accuracy of non-ECG gated CT with echocardiography in measuring left atrial and aortic root dimensions to detect left atrial enlargement (LAE) in 123 dogs. They assumed that LA/Ao ratio of 1.6 was the dividing line between a normal and enlarged left atrium, and they assumed that the echo measurements were "gold standard". They concluded that the LA/Ao ratio from CT scsns "wsa not a sensitive discriminator for LAE in dogs", and that, "Overall, the method evaluated in this study was not able to reliably detect dogs that have echocardiographically confirmed LAE and, as such, appears to have low clinical utility."
RETURN TO TOP
-- electrocardiography (ECG or EKG)
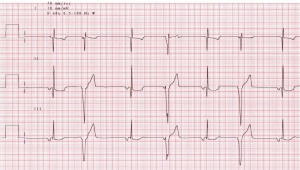 Electrocardiography (ECG or EKG) is a diagnostic tool
that measures and records the heart's electrical activity. Multiple,
advanced resting electrocardiographic techniques have been applied to humans
to detect cardiac diseases before onset of symptoms or changes in the
standard ECG. It is considered the gold standard for detecting arrhythmias.
Electrocardiography (ECG or EKG) is a diagnostic tool
that measures and records the heart's electrical activity. Multiple,
advanced resting electrocardiographic techniques have been applied to humans
to detect cardiac diseases before onset of symptoms or changes in the
standard ECG. It is considered the gold standard for detecting arrhythmias.
In a June 2011 study by Slovenian and Danish researchers, they were able to use advanced ECG to predict the severity of mitral regurgitation in dogs with MVD. They reported:
"Our results indicate that for a cut-off criteria of MR [mitral regurgitation] 50% jet the five selected ECG parameters could predict the severity of MR caused by MMVD in CKCSs with sinus rhythm with sensitivity 65% (78% with age inclusion) and specificity 98% (92% with age inclusion) (P < 0.05)."
In a September 2016 article which included CKCSs with MVD, researchers found that sinus rhythm is the most common rhythm disturbance in dogs with MVD, compared to supraventricular rhythm in dogs with dilated cardiomyopathy. They also found that the duration of QRS complex (Q-wave; R-wave; and J-point of S-wave)was significantly higher in dilated cardiomyopathy group.
In an August 2017 article, Spanish researchers found that electrocardiographic P wave-related parameters (classical and newly described) had low sensitivity (range=52.3% to 77%; median=60%) and low to moderate specificity (range=47.2% to 82.5%; median 56.3%) for the prediction of left atrial enlargement. Note that newly described P wave-related parameters include P wave area, Macrux index and mean electrical axis, and classical P wave-related parameters are voltage and duration of P wave.
In a September 2019 article, researchers found that there is a gradual increase in the ECG value of intrinsicoid deflection (DI) as the stage of MVD progresses from Stage B1 to B2 to C, associated with left ventricular overload. They concluded that DI measurments can be use to classify MVD-affected dogs.
RETURN TO TOP
-- artificial intelligence
Artificial intelligence (AI) is the term which describes the use of scientific algorithms applied to data obtained from formats of cardiovascular imaging to predict the progression of heart disorders in humans and animals. In the field of studying mitral valve disease in dogs, AI algorithms have been applied to data from auscultations, x-rays, and echocardiograms. A machine-learning algorithm is a set of rules or processes used by an AI system to perform functions. Machine-learning is the core of most AI applications. An AI program called a "neural network" is a formula designed to solve specific tasks, with gating recurrent unit cells used to selectively update the network as it performs its functions. For example, a neural network can be used to process echocardiographic measurements in the same fashion as would veterinary echocardiographers in their practices.
In a September 2023 article, Italian, Polish, and Swiss cardiologists applied an AI algorithm to heart x-rays of 1,242 dogs diagnosed with MVD, in an effort to match the x-ray diagnoses with the 2019 ACVIM definitions of Stages B1, B2, C, and D of MVD. They found that their algorithm had "high accuracy" in predicting Stages B1, C, and D, but not Stage B2. They found that "a significant number", 23.6%, of the dogs classified as being in Stage B2 by echocardiograms were "misclassifed" by the AI algorithm as Stage B1. They termed the accuracy level for detecting Stage B2 dogs as only "moderate".
In a February 2024 article, a world-wide team of veterinary echocardiographers -- cardiologists and radiographers -- compared their measurements of the left ventricles (LV) in 461 canine echocardiograms, including those of 39 cavaliers, with an AI program (a neural network) designed to process LV data from echocardiograms in the same fashion as do the veterinary echocardiographers in their practices. They concluded that: "An artificial intelligence network can be trained to adequately measure linear LV dimensions, with performance indistinguishable from that of experts."
In an August 2024 article, veterinary researchers from Italy, Poland, and Switzerland developed a "heart convolutional neural network" (CNN) for the purpose of predicting the stage of MVD (B1, B2, C, D) and MINE scores of canine patients, using data from x-rays. X-rays of the hearts of 556 dogs were examined. The overall precision in predicting MVD from the lateral x-rays, according to the ACVIM guidelines, was 67%, with a precision of healthy: 68%, Stage B1: 69%, Stage B2: 51%, Stage C: 73%, and Stage D: 33% respectively. The overall precision in predicting MVD from the x-rays, according to the MINE score was 60%, with a precision of healthy: 59%, Stage B1: 66%, Stage B2: 30%, Stage C: 69% and Stage D: 40% respectively.
In an October 2024 article, a team of UK veterinary researchers and AI engineers reported on the design of a machine-learning algorithm capable of accurately detecting and grading mitral valve murmurs using electronic stethoscope recordings. In this study, the 15 second digitally recorded sounds of the left apex, left base, and right-sided auscultation positions over the heart of each dog were transformed into a normalized log-spectrogram time-frequency representation, followed by a bi-directional recurrent neural network with gated recurrent unit cells trained to predict the presence of a murmur. A neural network is a formula designed to solve specific tasks, with gating recurrent unit cells used to selectively update the network as it performs its functions. Of the 343 dogs diagnosed with mitral valve disease (MVD or MMVD) in this study, the cavalier King Charles spaniel was the most common breed, consisting of 88 dogs (25.7%). All dogs received a full physical and echocardiographic examination by a cardiologist to grade any murmurs and identify the heart disease. The AI algorithm, based upon one designed for heart murmur detection in humans, was fine-tuned with dog data to predict the cardiologist's murmur grade from the audio recordings of the electronic stethoscopes. The investigators reported that the algorithm detected murmurs of any grade with a sensitivity of 87.9% and a specificity of 81.7%. The predicted grade exactly matched the cardiologist's grade in 57% of the recordings. The algorithm's prediction of loud or thrilling murmurs effectively differentiated between stage B1 and B2 MVD, with a sensitivity of 81.4% and a specificity of 73.9%. They concluded that a machine-learning algorithm trained on humans can be successfully adapted to grade heart murmurs in dogs caused by common cardiac diseases and assist in differentiating preclinical MVD.
RETURN TO TOP
-- cardiac catheterization
Elevated left atrial pressure (LAP) is the most common sign of MVD in affected dogs. Cardiac catheterization is the most accurate method of measuring the elevation of LAP. However, catheterization is very invasive and requires anesthesia. In this September 2004 article, echocardiographic estimation of LAP, by calculating the E-peak velocity (E-peak) to Ea-peak ratio (E/Ea ratio) was found to correlate well with the "mean" (average) LAP in dogs.
Cardiac catherization of the right side of the heart is consdered the "gold standard" for diagnosing pulmonary hypertension (PH) -- increased blood pressure in the lungs' arteries -- because it measures the pulmonary arterial pressure (PAP) directly in the lungs' blood vessels.
RETURN TO TOP
-- mass spectrometry
 Mass spectrometry is a technique that identifies the chemical
composition of a sample of a compound based on the mass-to-charge ratio
of electrically charged particles. A mass spectrometer (right) plots the
mass-to-charge (m/z) ratio of compounds in a sample (usually serum or
saliva). The sample is
vaporized into a gas and then ionized. The ions are focused into an ion
beam which passes through a magnetic field which bends the beam,
deflecting the ions' components onto a spectrum based upon their weight
(mass) and degree of charge.
Mass spectrometry is a technique that identifies the chemical
composition of a sample of a compound based on the mass-to-charge ratio
of electrically charged particles. A mass spectrometer (right) plots the
mass-to-charge (m/z) ratio of compounds in a sample (usually serum or
saliva). The sample is
vaporized into a gas and then ionized. The ions are focused into an ion
beam which passes through a magnetic field which bends the beam,
deflecting the ions' components onto a spectrum based upon their weight
(mass) and degree of charge.
Two specific types of mass spectrometry are used in diagnosing patterns of peptides -- hormones manufactured and secreted by areas of the heart -- to identify peptide mass, called "peptide barcodes", which can be used as diagnostic tools to recognize certain diseases. These MS techniques are called "matrix assisted laser desorption time of flight mass spectrometry" (MALDI-TOF MS) and "liquid chromatography tandem mass spectrometry" (LC-MS/MS).
In a February 2017 article on a study of twelve cavaliers, an Italian team used MALDI-TOF MS to identify eight serum proteins which were differentially expressed between groups of moderately (M) and severely (S) affected CKCSs with MVD and a control group of healthy cavaliers. These proteins were found to be significantly related to the progression of the disease. See the "serum protein" section of this article for details of this study.
In a December 2020 article, a team of Thai researchers used these two MS methods to distinguish biomarkers between dogs with normal hearts and MVD-affected dogs in the various stages (B and C) of MVD and those with pulmonary hypertension (PH) in addition to MVD. They enrolled 59 dogs -- none of which were cavalier King Charles spaniels -- and divided them into five groups (11 normal dogs; 16 in Stage B2 MVD; 5 in Stage B2 and having PH; 11 in Stage C;, and 16 in Stage C with PH). Among these dogs, six amino acid sequences of peptide candidates were identified as candidate biomarkers which may be involved in the pathogenesis of PH and MVD.
RETURN TO TOP
-- natriuretic peptides tests (ANP and BNP)
Bottom Line: Medical treatment for MVD should never be based solely upon a biomarker test.
There has been much research into attempting to diagnose MVD, and more particularly, to diagnose the onset of heart failure (CHF) in dogs, by measuring "cardiac biomarkers". These biomarkers are biological subsances of many kinds found inthe blood plasma, which when measured may aid in the screening, monitoring, and prediction of outcome of a disease. They all are derived from minimally invasive techniques from the patients. Biomarkers of MVD all pertain to conditions of the MVD-affected dog's heart -- its stress, injury, enlargemen, and other alterations.
Natriuretic peptides, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), are hormones manufactured and secreted by areas of the heart.
• ANP is responsible for the regulation of blood pressure and body fluid homeostasis. It secretes from the two atria chambers of the heart (left and right chambers) as the walls of either of these chambers is caused to stretch due to the progression of MVD.
• BNP has diuretic, vasodilatory, and anti-remodeling properties which is produced by myocardial cells in response to heart failure. It secretes from the two ventricle chambers of the heart (left and right chambers) as the walls of either of these chambers is caused to stretch due to the progression of MVD.
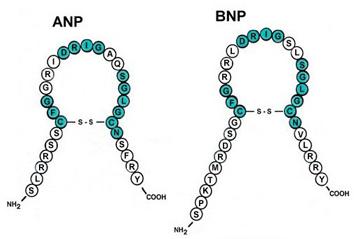 They both can be used as biomarkers specifically to detect cardiac
disease and congestive heart failure in dogs (and cats). Heart wall
stress in response to volume overload increases the production of
natriuretic peptides such as NT-proBNP from cardiomyocytes. Therefore,
increased measured quantities of natriuretic peptides can indicate
enlargement of the heart due to MVD.
However, ANP is less stable than BNP and there for more difficult to
monitor as a biomarker.
They both can be used as biomarkers specifically to detect cardiac
disease and congestive heart failure in dogs (and cats). Heart wall
stress in response to volume overload increases the production of
natriuretic peptides such as NT-proBNP from cardiomyocytes. Therefore,
increased measured quantities of natriuretic peptides can indicate
enlargement of the heart due to MVD.
However, ANP is less stable than BNP and there for more difficult to
monitor as a biomarker.
A test of natriuretic peptides measures the quantity of the natriuretic peptides in the dog's blood. Elevated levels of these natriuretic peptides in the blood may be directly related to heart defects, and natriuretic peptides in the blood become elevated only after the heart has to pump harder to compensate for the disorder. In particular, BNP is secreted by the left ventricle in response to heart wall stretching or stress.
A 2003 study (conducted by Drs. Kristin A. MacDonald, Mark D. Kittleson, Coralie Munro, and Philip Kass of the University of California at Davis) has shown a positive correlation between BNP and heart disease and CHF in dogs. In that study, BNP increased with the progressively increased severity of mitral valve disease and CHF. For every 10-pg/mL increase in BNP, the 2003 study's dogs' mortality rate increased approximately 44% over the four months of the study. In a 2005 study, Drs. William E. Herndon, Justine A. Lee, Kenneth J. Drobatz, and Matthew J. Ryan concluded that "With further investigation, this new BNP assay may someday provide a widely available noninvasive diagnostic test with rapid turnaround time to help diagnose and/or treat heart disease and congestive heart failure in the dog."
However, in earlier studies (1994 and 1997) conducted by Drs. Jens Haggstrom, Kjerstin Hansson, Clarence Kvart, and others, the researchers have suggested that BNP levels in cavaliers with mitral regurgitation did not rise as dramatically as in humans, and that N-terminal (NT)-proANP (NT-proANP) may better reflect the severity of mitral regurgitation in cavalier King Charles spaniels than NT-proBNP tests.
Four trademarked names for NT-proBNP tests are Canine CardioCare (Veterinary Diagnostics Institute), Canine VetSign CardioSCREEN (Guildhay Ltd.), Cardiopet proBNP (IDEXX Laboratories), and Antech Cardio-BNP (Antech Diagnostics). There have been studies showing the effectiveness of these types of tests for dogs suffering from asymptomatic occult dilated cardiomyopathy (DCM), which is not the same disorder as MVD and is not known to be a genetic problem for cavalier King Charles spaniels.
Whichever test (NT-proBNP or NT-proANP) is found to be more accurate for detecting MVD, it is believed by some researchers that the test may be useful in assisting examining veterinarians in deciding whether or not detected heart murmurs are innocent or are pathologic in nature. However, in a 2007 study of 54 CKCSs by Drs. Tarnow, Pedersen, Kvart, and others from Denmark and Sweden, they found that "Natriuretic peptides are elevated in cavalier King Charles spaniels with congestive heart failure but not in dogs with clinically inapparent mitral valve disease."
In a May 2008 report by Drs. Mark A. Oyama, Philip R. Fox, John E. Rush, Elizabeth A. Rozanski, and Michael B. Lesser of 119 dogs, they found that "Serum NT-proBNP concentration was significantly higher in dogs with cardiac disease than in control dogs, and a serum NT-proBNP concentration > 445 pmol/L could be used to discriminate dogs with cardiac disease from control dogs with a sensitivity of 83.2% and specificity of 90.0%. In dogs with cardiac disease, serum NT-proBNP concentration was correlated with heart rate, respiratory rate, echocardiographic heart size, and renal function." They concluded that, "For dogs with cardiac disease, serum NT-proBNP concentration could be used to discriminate dogs with and without radiographic evidence of cardiomegaly and dogs with and without congestive heart failure." And that, "Results suggested that serum NT-proBNP concentration may be a useful adjunct clinical test for diagnosing cardiac disease in dogs and assessing the severity of disease in dogs with cardiac disease."
In a May 2009 report from Sweden, the researchers concluded: "Plasma concentrations of the natriuretic peptides measured at re-examination could predict progression in regurgitant jet size."
In an August 2009 report from Japan, the researchers concluded, "These findings could indicate that plasma NT-proBNP concentration was significantly associated with the severity of heart failure due to mitral valve insufficiency in dogs. Further investigation is required to determine factors other than heart failure affecting plasma NT-proBNP concentration."
In a 2012 study of 1,134 dogs, including 37 cavaliers, Stephen J. Ettinger, Giosi Farace, Scott D. Forney, Michelle Frye, and Andrew Beardow concluded that "This biomarker [NT-proBNP] may be a useful tool for staging of cardiac disease and identifying cardiac-related coughing or dyspnea in this species."
In a 2013 study of 36 dogs, none being CKCSs, a team of Japanese researchers concluded: "These results indicated that plasma ANP rose with an increase in the volume overload of the left side of the heart. Plasma ANP discriminated cardiomegaly from non-cardiomegaly caused by asymptomatic MMVD. We conclude, therefore, that plasma ANP concentrations may be a clinically useful tool for early diagnosis of asymptomatic MMVD in dogs."
In an April 2013 report, a team of German veterinary cardiologists studied 352 dogs and found that: "NPs [natriuretic peptides] in canine MMVD are useful to discriminate between asymptomatic dogs and dogs with CHF. Due to a large overlap of NP-concentrations between the groups, NPs do not seem to be useful to differentiate between dogs in stages B1 and B2. Interpretation of NT-proBNP and proANP values should include consideration of sex-specific differences."
In a March 2014 Swedish study of 535 healthy dogs of nine breeds, including 34 cavaliers, researchers found that CKCSs and German shepherds had the highest median ANP concentrations, twice the median concentration in the breed with the lowest concentration, the Doberman Pinscher. Most importantly from that 2014 study, the researchers found that the levels of NT-proBNP concentrations are breed-specific. In other words, there can be no species-wide reference level for NT-proBNP concentrations in healthy dogs.
In a July 2014 report, a Swedish/Finnish/Danish team examined 78 cavaliers with MVD and found that the risk of CHF increased with NT-proANP concentrations above 1000 picomoles per liter (pmol/l). In an October 2014 study of 291 dogs, including 38 cavaliers, researchers once again found that plasma NT-proBNP concentrations were higher in dogs in congestive heart failure (CHF) than in dogs with non-cardiac respiratory distress.
In a January 2017 report, researchers focused upon the inherent variability of NTproBNP in all dogs -- both healthy and MVD-affected -- and how those random variations, called biologic variability, affects the accuracy of population-based reference ranges of NTproBNP readings. They measured NTproBNP in healthy dogs and dogs in stages B1, B2, and C of mitral valve disease, to estimate biologic variability and calculate an Index of Individuality (IoI) for each dog. Of the 28 MVD-affected dogs in the study, 14 -- 50% -- were cavalier King Charles spaniels, along with 10 healthy control dogs. They found that MVD-affected dogs had a lower inherent variability of NTproBNP compared to healthy dogs, and that, as a group, MVD-dogs required a change of 58.2% for NTproBNP to be outside the range of inherent biologic variability and indicate a change in disease. They determined that population-based reference ranges for NTproBNP may have limitations. They stated that:
"It is important for veterinarians to have an understanding of how much a biomarker value could change over time due to randomness vs. disease progression in an individual dog. ... Monitoring serial individual changes in NTproBNP values may be clinically relevant in addition to using population-based reference ranges to determine changes in disease status."
In a September 2018 article, a team of USA and UK veterinary cardiologists tested a treatment schedule for MVD-affected dogs in heart failure (CHF) based upon the levels of the NT-proBNP concentration in each dog's bloodstream. The goal of their study was to reduce NT-proBNP because of prior studies showing that such reductions improves outcomes in human CHF patients. Twenty-six dogs were in the study, which included five cavaliers -- the largest number of any breed -- with four examinations over a 21 day period. The dogs were divided into three groups, all three of which were in stable condition based upon routine treatments for CHF (e.g., furosemide, hydrochlorothiazide, spironolactone). Groups were:
Group 1: Baseline NT-proBNP was <1500 pmol/L. No dose adjustments were made to the current treatment.
Group 2: Baseline NT-proBNP was >1500 pmol/L. Dogs were eligible for prespecified medical treatment escalation on visit 0 or visit 1 if the serum creatinine concentration was <3.0 mg/dL. If creatinine was >3.0 mg/dL, data from the dog would be included in the analysis, but the dog would be ineligible for further treatment escalation."Treatment was escalated according to a prespecified plan as follows: if the current furosemide dose was <6 mg/kg/day, this dose was increased by 50%; if the current furosemide dose was >6 mg/kg/day, a combination of hydrochlorothiazide (1 mg/kg q24h) and spironolactone (1 mg/kg q24h; Aldactazide, Pfizer, New York) was added; if the current dose of furosemide was >6 mg/kg/day and the dog was already receiving hydrochlorothiazide and spironolactone the daily pimobendan dose was increased by 50%-100%."Group 3: Control Group. Baseline NT-proBNP was >1500 pmol/L but received no adjustment in treatment over the 21 day period.
The researchers found that NT-proBNP decreased significantly in group 2, but not in groups 1 or 3. They concluded that the "application of a prespecified treatment escalation algorithm in dogs with recent history and treatment of 1st time CHF secondary to MMVD results in a decrease in plasma NTproBNP concentrations", and that "Use of this treatment escalation algorithm allows effective targeting of treatment for CHF in dogs against an objective criterion."
What this means is that natriuretic peptides may be useful in both predicting the rapidity of the progression of MVD -- the "prognostic potential" -- and also signaling when medical treatment should be increased -- what veterinarians refer to as the "titration of therapy".
In an October 2023 article, a team of veterinary cardiologists performed various cardiac tests -- vertebral heart size (VHS), N-terminal pro B-type natriuretic peptide blood test (NT-proBNP), vertebral left atrial size (VLAS), and electrocardiography -- on 226 cavaliers to determine which tests best predict echocardiography findings of Stage B1 or Stage B2 (LA/Ao>1.6 and LVIDdN>1.7) of mitral valve disease, based upon the 2019 ACVIM Consensus Statement. Their stated goals were to develop breed-specific cut-offs for individual screening tests and develop predictive models in combination for identifying Stage B2 cavaliers in cases in which no echocardiographic examinations are available. As for NT-proBNP reference ranges, they reported that for CKCSs in Stage B1, the reference Interval was 515.2-917.3; range 250.0-2208.4. For cavaliers in Stage B2, the Reference Interval was 960.4-1972.8; range 478.8-3656.8. They stated that an NT-proBNP 1138 pmol/L had high specificity for predicting Stage B2.
The Bottom Line on natriuretic peptides: It appears that veterinary cardiologists and other cardio-specialists should be quite capable of detecting mitral valve regurgitation murmurs and distinguishing between them and flow murmurs or other innocent varieties of heart murmurs. Since ANP and BNP in the blood becomes elevated only after the heart has to pump harder to compensate for the disorder, the question then is: When does the heart start working so hard that BNP levels start to go up? In the cavalier King Charles spaniel's version of heart defects -- mitral valve disease due to deteriorating valve flaps -- there are no immediate external symptoms. It is not yet clear from research studies thus far, as to whether the heart becomes labored enough to produce increased levels of BNP before auscultation is able to detect the murmurs from minimal backflow of blood leaking through the mitral valve flaps. Advocates of ANP and BNP testing do represent that that studies of ANP and BNP and cardiomyopathy show that ANP and BNP are elevated before the onset of signs and murmur. But it does not yet appear that ANP or BNP testing necessarily is an any earlier warning system for MVD than auscultation.
Bolstering this viewpoint is the comment by Dr. Jennifer L. Garcia in "The NT-proBNP assay: A portent of heart health." in dvm360:
"For conditions such as mitral valve disease, this test may be of limited value because a diagnosis can be readily made by thorough auscultation and documentation of a heart murmur. In these cases, the assay also has limited utility in determining disease severity; thoracic radiography is preferred."
For an added twist, in a June 2016 abstract, Australian researchers have added corin to this list. Corin is a gene on chromosome 4p13-p12 that encodes a serine-type endopeptidase which converts non-functional propeptide NPPA into active atrial natriuretic peptide hormone and processes pro-NPPB, the B-type natriuretic peptide. In the abstract, the concluded that:
"A lack of an increase in corin expression or activation can be postulated to be a possible important pathophysiological mechanism responsible for the natriuretic peptide paradox in dogs with CHF, and warrants further investigation."
One possible uniquely valuable use for natriuretic peptides tests is if the dog is approaching heart failure (HF) without any symptoms. In that case, natriuretic peptides tests, combined with "Left Chambers on Aorta ratio" greater than 4,5, the veterinarian may begin administering ACE inhibitors, pimobendan, and other drugs immediately even though the dog is asymptomatic. See Dr. Gerard Le Bobinnec's proposal in this report to the 2010 WSAVA Congress. See, also, the 2012 report of the PREDICT Cohort Study, which found that measurements of left heart size (using the "left atrial to aortic root dimension ratio [LA:Ao]") and plasma NT-proBNP concentration independently estimate risk of first-onset of CHF in dogs with MVD. It correctly predicted first-onset of CHF in 72.5% of cases out of 82 dogs, which included cavaliers.
Dr. Oyama has stated that natriuretic peptide tests may also be useful to properly diagnose a dog known to suffer from congestive heart failure and also is in respiratory distress. He said that, "When dogs come into veterinary hospitals in respiratory distress, it's sometimes difficult to know if they are having a respiratory or heart problem. Such a test could speed effective treatment and also help decide if a dog should be referred to a veterinary cardiologist before undergoing more expensive testing."
Prof. Melanie Hezzell stated in her June 2025 article that:
"There is significant day-to-day variation in plasma NT-proBNP concentrations, as expected for a neurohormone; a measurement must therefore double before the change can be considered significant. Circulating NT-proBNP concentrations have been shown to double on average every 13 months in dogs that die due to MMVD. Serial monitoring of plasma NT-proBNP concentrations can therefore be useful in identifying dogs with progressive disease."
Dr. Adrian Boswood of the Royal Veterinary College has suggested that the NT-proBNP assay may have some value in predicting the future life span of dogs in congestive heart failure. He is participating in an on-going study, called the VetCompass DMVD project, which reportedly aims to identify factors associated with survival of MVD-affected dogs, by evaluating basic clinical measurements and cardiac biomarker levels (NT-pro-BNP and cardiac troponin I).
Finally, the September 2018 study (referenced above) of periodic testing of NT-proBNP levels after the onset of CHF may be useful in regulating the dosages of diuretics. However, in that study, the dogs' blood plasma was tested four times during a three week period, which is not particularly practical for most such patients.
RETURN TO TOP
-- other cardiac biomarkers
Bottom Line: Medical treatment for MVD should never be based solely upon a biomarker test.
 A "cardiac biomarker" is a substance manufactured by the dog and
usually released into the dog's blood system, which is heart specifc and
released during the progression of a disease, in proportion to the
extent of damage to the heart being caused by that disease.
A "cardiac biomarker" is a substance manufactured by the dog and
usually released into the dog's blood system, which is heart specifc and
released during the progression of a disease, in proportion to the
extent of damage to the heart being caused by that disease.
There has been much research into attempting to diagnose MVD, and more particularly, to diagnose the onset of heart failure (CHF) in dogs, by measuring cardiac biomarkers with varying degrees of success. Above, we have described the main cardiac biomaker for MVD, plasma concentrations of the natriuretic peptides: atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP).
Other cardiac biomarkers include:
• cardiac troponin I (cTnI), and high-sensitivity cardiac troponin I
(hscTnI)
• C-reactive protein (CRP)
•
sodium-calcium exchanger (NCX-1)
•
aldosterone
concentration (UAC)
•
serum serotonin (serum 5HT)
• antidiuretic hormone (ADH)
•
histamine
concentration
•
enzymes and growth factors
•
transforming growth factor beta (TGF-B)
•
Galectin-3 (Gal-3)
• vitamin D
•
serum cystatin-C (Cys-C) and symmetric dimethylarginine (SDMA)
•
oxidative stress
•
serum fatty acids
•
serum leptin and
adipokines
• anemia
•
desmin, vimentin, periostin,
caspase-3
• serum
proteins
• serum homocysteine
• serum
nitrotyrosine
• heart-fatty
acid binding protein (hFABP)
•
soluble ST2 (sST2)
•
adiponectin
•
serum iron
concentration
•
humanin (HN)
Most of the protein-based biomarkers are identified using plasma proteomics.
---cardiac troponin I (cTnI)
Several studies of cardiac troponin I (cTnI) have shown increases in cTnI concentrations in dogs with poor prognoses, and that cTnI has potential in assessing the prognosis and severity of MVD and enlargement of the heart. See the 2009 study, the January 2010 study, the June 2010 study, and the April 2013 study. In a February 2012 study, high-sensitivity cardiac troponin I (hscTnI), in combination with N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations, has been examined. The researchers concluded that:
"Survival times were shortest in dogs in which both serum hscTnI and NT-proBNP were increased. hscTnI and NT-proBNP increased more rapidly in dogs that died of cardiac disease. Conclusions and Clinical Importance: Serum hscTnI has prognostic value in dogs with DMVD. Measurement of NT-proBNP and hscTnI is prognostically superior to measuring either alone. Serial measurement strategies provide additional prognostic information."
The VetCompass DMVD project is an on-going study which reportedly aims to identify factors associated with survival of MVD-affected dogs, by evaluating basic clinical measurements and cardiac biomarker levels (NT-pro-BNP and cardiac troponin I).
In an April 2017 article, a team of Texas A&M veterinary school investigators applied the concept of biologic variability (BV) testing to concentrations of cardiac troponin I (cTnI) in periodic blood samples of 10 healthy and 28 MVD-affected dogs (including 14 [50%] cavaliers). They noted that the use of a standard sensitivity cTnI assay was insufficiently sensitive to quantify and study changes in cTnI concentration in both healthy dogs and MVD-affected dogs in their study. They argue that long-term serial measurements of cTnI changes helped predict the MVD end-points of onset of heart failure and death. They suggest that such periodic testing may provide more information for predicting survival time than a single measurement.
In a February 2020 article, a team of Japanese researchers studied 316 MVD-affected dogs, including 41 cavaliers (13%) to determine if atrial natriuretic peptide (ANP) and/or cardiac troponin I (cTnI) concentrations could diagnose MVD in dogs and also determine the stage of MVD (B1, B2, or C) of the dogs. They concluded that ANP concentrations appeared to be useful for detecting dogs in Stage B1 and Stage B2, and that both ANP and cTnI were useful in assessing whether the dogs were in heart failure (Stage C), but that neither test should be used independently of echocardiography to diagnose MVD.
See, also, this July 2023 article describing that "Cardiac troponin I (cTnI) is considered the gold standard biomarker for myocardial injury and shows a high degree of homology between humans and dogs."
RETURN TO TOP
---C-reactive protein (CRP)
In a 2006 study, researchers found that, compared with controls, dogs with chronic valvular disease had higher plasma concentration of C-reactive protein (CRP). In a 2015 report, researchers found a "statistically significant difference" in CRP concentrations between MVD-affected dogs as their MVD progressed from asymptomatic to advanced heart failure. They noted that CRP concentrations were "significantly higher" in the asymptomatic group, and that "Differences in CRP concentrations between clinical stages of MVD suggest a clinically and therapeutically relevant inflammatory component."
However, in a December 2015 report, Swedish and Danish cardiologists studied 188 dogs with varying degrees of MVD, to determine whether CRP concentrations are associated with the severity of MVD, using an automated canine-specific high-sensitivity CRP assay called "Gentian hsCRP". They found that serum CRP concentrations were mildly increased in dogs with congestive heart failure due to MVD, but that the severity of MVD in asymptomatic dogs showed no association with serum CRP concentrations.
RETURN TO TOP
---sodium-calcium exchanger (NCX-1)
Sodium-calcium exchanger (NCX-1) is being examined as an alternative to natriuretic peptides because NCX-1 appears to better differentiate between heart failure and renal failure. See this 2010 South Korean report. Also, leptin, a protein produced by fat tissues and associated with canine body fat, was found in an August 2011 UK report to be more highly concentrated in dogs with congestive heart failure.
RETURN TO TOP
---aldosterone concentration (UAC)
In a November 2012 report, researchers found that left ventricular heart enlargement in dogs with MVD is associated with a decrease in the serum concentration of a marker of collagen type III turnover, and an increase in urinary aldosterone concentration (UAC). They also reported that both serum N-terminal procollagen type III concentration and UAC were higher in cavalier King Charles spaniels than in other breeds when other measured variables were controlled for.
However, in an April 2013 study of 50 dogs (including 20 CKCSs), the researchers reported that:
"Cardiac fibrosis and arteriosclerosis in dogs with MMVD are reflected by circulating cTnI [cardiac Troponin-I] concentration, but not by aldosterone concentration or renin activity. Cardiac troponin I could be a valuable biomarker for myocardial fibrosis in dogs with chronic cardiac diseases." (Emphasis added.)
RETURN TO TOP
---serum serotonin (serum 5HT)
In
a July 2013 study of 120 dogs,
including 92 cavaliers, by an international team of
cardiologists, the researchers found that cavaliers had higher
concentrations of serum serotonin (serum 5HT) than other
breeds not predisposed to mitral valve disease, and that serum 5HT
concentrations decreased with increased left atrial enlargement. They
concluded that, "the finding of higher serum 5HT concentrations in dogs
predisposed to MMVD (CKCS) and dogs with mild MMVD suggests that alterations
in 5HT signaling might play a role in progression of early stages of MMVD."
In a 2009 study, some
of the same researchers found, "Dogs with DMVD had significantly higher
serum 5HT concentrations when compared with large breed control dogs.
Healthy CKCS dogs had significantly higher serum 5HT concentrations than
other healthy dogs predisposed to DMVD."
In a January 2013 interview with AKC's Canine Health Foundation, board certified cardiologist Dr. Mark Oyama of University of Pennsylvania's veterinary school, said:
"Our research involving serotonin and other pathways involved in the development and progression of disease are ultimately targeted towards discovering the underlying abnormalities that produce mitral valve disease in dogs. If serotonin and other pathways contribute to disease formation blockade of these pathways could result in reduction in disease development and progression."
In a July 2014 report, the international team found that platelet serotonin was elevated in cavaliers compared to other breeds, and that serotonin in left ventricular myocardial and mitral valve leaflet tissue in deceased MVD dogs was elevated compared to dogs which died without cardiac disease. See also this September 2014 report finding serotonin concentration high in cavaliers.
In a September 2016 abstract, Danish and Swedish cardiologists reported their preliminary study of 63 cavaliers and 17 control dogs to determine if 5-Hydroxyindoleacetic acid (5-HIAA), a serotonin metabolite excreted in urine, is associated with MVD severity in the CKCS. They concluded that the preliminary data indicated that urinary 5-HIAA concentrations are dependent on the sex, but not the age of the dog, but that further analyses are necessary to draw final conclusions regarding the possible associations between urinary 5-HIAA concentrations and MVD severity in cavaliers. In a June 2019 article, the same team reported that they examined urinary excretions of serotonin (5-hydroxyindoleacetic acid [5-HIAA]) in 40 cavaliers, 11 without mitral valve disease (MVD) and 29 with MVD prior to heart failure (Stage C) to determine if urine concentrations of 5-HIAA were associated with MVD severity or blood platelet count or circulating serotonin. They concluded that while female cavaliers had higher urine 5-HIAA concentrations than males, 5-HIAA concentrations in CKCSs were not associated with MVD severity or blood platelet count or circulating serotonin.
In a December 2017 article, a team of human and veterinary researchers studied the relationship between serotonin receptors and mitral valve interstitial cells (MVICs) and leaflet remodeling in humans and cavalier King Charles spaniels. Mitral valve specimens from four deceased cavaliers with severe mitral valve prolapse (MVP) and mitral regurgitation and four normal controls showed that serotonin 5HT receptors (5HTRs) signaling contributes to MVP deterioration, in particular, 5HTR2B upregulation. While this was primarily a study of human MVP, it indicates a possible relationship between the upregulation of 5HTR2B in cavaliers and the progression of mitral valve deterioration. It also shows that a 5HTR2B antagonist -- a drug called LY 272015 hydrochloride -- reduces MVICs activation in humans and mice. Since the cavaliers in this study all were deceased, the application of LY 272015 to dogs was not part of this study. LY 272015 is a beta-carboline derivative drug developed by the Eli Lilly company which is known to act as a potent and selective antagonist at the serotonin 5-HT receptor.
RETURN TO TOP
---antidiuretic hormone (ADH)
Antidiuretic hormone (ADH) is produced by the hypothalamus and contributes to regulating blood pressure and blood plasma osmolality. High levels of ADH are believed to play a role in the development of congestive heart failure (CHF) in humans. For humans, an enzyme immunoassay (EIA) kit is used to quantify ADH levels. In a September 2013 study, a team of cardiologists from Oregon State University used EIA kits to compare the ADH concentrations in 6 healthy dogs and 12 with CHF. They researchers concluded that EIA kits can be used to determine ADH concentrations in dogs and that the dogs in CHF had significantly higher ADH concentrations than did the healthy dogs.
RETURN TO TOP
---histamine concentration
In a May 2014 report, Japanese cardiologists found that histamine concentration was higher in the population of dogs with MVD compared with the healthy controls.
---enzymes and growth factors
In an October 2014 study, researchers found that mitral valve and myocardial protein and gene expression of the enzymes matrix metalloproteinases (MMPs), their tissue inhibitors (TIMPs) and transforming growth factor-B (TGF-B) and plasma MMP and TGF-B concentrations appear to play a local role in the development of advanced MMVD.
In a July 2015 thesis, a Swedish veterinary student studied serum activity of matrix metalloproteinase-2, -9 and -14 in 66 dogs, including 49 cavaliers. He concluded that the activity of MMP-14 increases and the activity of MMP-9 decreases when systolic function is impaired; that activity of both enzymes vary during pathogenesis which may implicate that they are involved in different stages of disease progression; and that the study suggests that MMP-2 plays a minor role in dogs with MMVD.
In a March 2016 article, researchers examined the levels of cytokine expression in peripheral blood mononuclear cells (PBMC) of dogs with MVD (none were cavaliers) at different stages and in healthy dogs. They investigated potential relationships between cytokine expression and echocardiographic indices of MVD severity, left ventricular (LV) function and remodelling. For this purpose, IL-102 1, IL-6, IL-8, TGF-B1 and TNF-a peripheral blood mononuclear cells (PBMC) expression was measured. In the dogs with MVD, there were differences in IL-8 and TGF-B1 mRNA concentrations between the ACVIM stages of MVD (Stages B1, B2, and C). No statistically significant differences were detected between groups for IL-1a, IL-1B, IL-6, or TNF-a PBMC expression. IL-8 expression increased with increasing MVD severity and TGF-B1 expression was higher in asymptomatic dogs with echocardiographic signs of heart enlargement (Stage B2) than in all other groups. They concluded that their results could suggest the involvement of these cytokines at different stages of MVD.
In a December 2017 article, Brazilian researchers investigated the concentration of IL-1B, 4, 6, 10, TNF-a and CRP in MVD-affected dogs of ACVIM Stages B1, B2, and C, along with a control group of healthy dogs. The report finding: (1) the serum levels of interleukin 1B increases in MVD-affected dogs, and even higher levels are observed in dogs with MVD symptoms; (2) there is a significant correlation between heart enlargement and congestive heart failure and the circulating levels of IL-1B and IL-4; and (3) the serum levels of IL-6 and the left-ventricular shortening fraction are negatively correlated.
In a January 2020 article, board certified veterinary cardiologist Dr. Mark Oyama and others reviewed the aspects of cardiovascular pathology shared by humans and MVD-affected cavaliers. He focused upon the neurotransmitter serotonin (5HT) and transforming growth factor beta (TGF-B), which he describes as sharing a common ability to activate mitral valve interstitial cells in both dogs -- particularly cavaliers -- and humans. He states: "The dog, and in particular the CKCS breed, represents a model to further study 5HT and TGF-B mechanisms and to test novel therapies." His list includes:
• "One example is the Cavalier King Charles Spaniel (CKCS) breed, in which death due to heart disease comprises over 30% of all causes of death."
• "Beginning at the age of 3 years, the yearly incidence of MMVD in the CKCS is approximately 7% and rises to as high as 15% in subsequent years, such that virtually all dogs exhibit disease by the age of 10 years."
• "CKCS, a breed with extremely high prevalence of MMVD, are reported to have particularly increased serum 5HT levels."
• "5HT in platelet rich plasma was higher in healthy CKCS, as well as both CKCS and mixed breed dogs with MMVD vs. healthy mixed breed controls."
• "Of interest, the CKCS breed is also associated with an inherited thrombocytopenia and giant platelet disorder affecting approximately half of all CKCS dogs."
• "In addition to having macrothrombocytes, the platelet population in CKCS dogs with MMVD also contains at least one flow cytometry scatterplot subpopulation with a higher percentage of surface-bound 5HT-positive expression and activation, but the exact relationship of platelet morphology, ultrastructure, and function to MMVD requires further study."
In an April 2020 article, a University of Edinburgh team of veterinary researchers led by Dr. Brendan Corcoran examined the mitral valves of 30 euthanized MVD-affected dogs, including 8 cavalier King Charles spaniels (27%) to classify the valves according to MVD severity (Grade 0 - normal -- to Grade 4 - severely affected) and to identify all differentially expressed genes (DEGs) in all four such grades. By transcriptomic profiling they identified a total of 1,002 DEGs associated with a large number of gene families. Depending upon the grade of MVD, they found significant changes in gene expression intensity in especially ACTA2, HTR2B, MMP12, and CDKN2A. The TGFB1, TNF, IFGN genes were identified as the top up-stream regulators in the diseased valve samples. Of those, they found that TGFB1 appears to have the strongest associations with the disease. They concluded:
"In conclusion, despite differences in the total number of DEGs identified as canine MMVD pathology progresses over a lifetime, disease initiation, and progression appears to be primarily dependent on changes in TGFB signaling. This is the first description of the temporal and spatial expression of gene changes associated with naturally occurring myxomatous degeneration in any species. Other signaling pathways likely contribute to disease pathogenesis over time, with some becoming involved only at the stage of advanced disease. The factors that trigger the development of valve myxomatous degeneration are still unknown, but aberrant TGFB signaling appears to initiate and perpetuate the valve pathology characteristic of this disease in the dog."
RETURN TO TOP
---transforming growth factor beta (TGFB)
Transforming growth factor beta (TGFB) is a combination of growth factors which are secreted to support cellular growth in dogs and most all other mammals. The progression of MVD has been found to involve a transition of normal valve cells (qVICs) to an activated myofibroblast type of cell (aVICs), and this transition is dependent on abnormal TGFB signaling. The mechanisms which initiate and progress MVD development have been found to be due to changes in the TGFB signaling pathway.
The signaling of TGFB in cavaliers is suspected to be unique among canines, and theefore studies are on-going to examine this further. As of 2021, CavalierHealth.org has been providing financial grants to Dr. Brendan Corcoran at the The Royal (Dick) School of Veterinary Studies to investigate the cause and effect of TGFB in the CKCS breed.
In an October 2014 study, researchers found that mitral valve and myocardial protein and gene expression of the enzymes matrix metalloproteinases (MMPs), their tissue inhibitors (TIMPs) and transforming growth factor-B (TGF-B) and plasma MMP and TGF-B concentrations appear to play a local role in the development of advanced MMVD.
In a March 2016 article, researchers examined the levels of cytokine expression in peripheral blood mononuclear cells (PBMC) of dogs with MVD (none were cavaliers) at different stages and in healthy dogs. They investigated potential relationships between cytokine expression and echocardiographic indices of MVD severity, left ventricular (LV) function and remodelling. For this purpose, IL-102 1, IL-6, IL-8, TGF-B1 and TNF-a peripheral blood mononuclear cells (PBMC) expression was measured. In the dogs with MVD, there were differences in IL-8 and TGF-B1 mRNA concentrations between the ACVIM stages of MVD (Stages B1, B2, and C). No statistically significant differences were detected between groups for IL-1a, IL-1B, IL-6, or TNF-a PBMC expression. IL-8 expression increased with increasing MVD severity and TGF-B1 expression was higher in asymptomatic dogs with echocardiographic signs of heart enlargement (Stage B2) than in all other groups. They concluded that their results could suggest the involvement of these cytokines at different stages of MVD.
In a January 2020 article, Dr. Mark Oyama and others reviewed the aspects of cardiovascular pathology shared by humans and MVD-affected cavaliers. He focused upon the neurotransmitter serotonin (5HT) and transforming growth factor beta (TGF-B), which he describes as sharing a common ability to activate mitral valve interstitial cells in both dogs -- particularly cavaliers -- and humans. He states: "The dog, and in particular the CKCS breed, represents a model to further study 5HT and TGF-B mechanisms and to test novel therapies." His list includes:
• "One example is the Cavalier King Charles Spaniel (CKCS) breed, in which death due to heart disease comprises over 30% of all causes of death."
• "Beginning at the age of 3 years, the yearly incidence of MMVD in the CKCS is approximately 7% and rises to as high as 15% in subsequent years, such that virtually all dogs exhibit disease by the age of 10 years."
• "CKCS, a breed with extremely high prevalence of MMVD, are reported to have particularly increased serum 5HT levels."
• "5HT in platelet rich plasma was higher in healthy CKCS, as well as both CKCS and mixed breed dogs with MMVD vs. healthy mixed breed controls."
• "Of interest, the CKCS breed is also associated with an inherited thrombocytopenia and giant platelet disorder affecting approximately half of all CKCS dogs."
• "In addition to having macrothrombocytes, the platelet population in CKCS dogs with MMVD also contains at least one flow cytometry scatterplot subpopulation with a higher percentage of surface-bound 5HT-positive expression and activation, but the exact relationship of platelet morphology, ultrastructure, and function to MMVD requires further study."
In an April 2020 article, a University of Edinburgh team of veterinary researchers led by Dr. Brendan Corcoran examined the mitral valves of 30 euthanized MVD-affected dogs, including 8 cavalier King Charles spaniels (27%) to classify the valves according to MVD severity (Grade 0 - normal -- to Grade 4 - severely affected) and to identify all differentially expressed genes (DEGs) in all four such grades. By transcriptomic profiling they identified a total of 1,002 DEGs associated with a large number of gene families. Depending upon the grade of MVD, they found significant changes in gene expression intensity in especially ACTA2, HTR2B, MMP12, and CDKN2A. The TGFB1, TNF, IFGN genes were identified as the top up-stream regulators in the diseased valve samples. Of those, they found that TGFB1 appears to have the strongest associations with the disease. They concluded:
"In conclusion, despite differences in the total number of DEGs identified as canine MMVD pathology progresses over a lifetime, disease initiation, and progression appears to be primarily dependent on changes in TGFB signaling. This is the first description of the temporal and spatial expression of gene changes associated with naturally occurring myxomatous degeneration in any species. Other signaling pathways likely contribute to disease pathogenesis over time, with some becoming involved only at the stage of advanced disease. The factors that trigger the development of valve myxomatous degeneration are still unknown, but aberrant TGFB signaling appears to initiate and perpetuate the valve pathology characteristic of this disease in the dog."
For more information about Dr. Corcoran's research into TGFB signaling, go to the Cell Transitions section, below.
RETURN TO TOP
---Galectin-3 (Gal-3)
In an April 2015 abstract and a January 2016 article, Thai researchers report finding that MVD-affected dogs have more cardiac fibrosis compared to normal dogs, and that plasma Galectin-3 (Gal-3) concentration was increased in MVD-dogs. They concluded that Gal-3 expression in cardiac muscle was associated with cardiac fibrosis, and that tissue Gal-3 is a candidate of fibrosis biomarker in MVD, but that further investigation of associations between plasma Gal-3 and myocardial fibrosis is necessary.
In a June 2017 abstract, Brazilian investigators sought to establish the utility of Gal-3, isolated or in association with Type B natriuretic pro-peptide (NTproBNP) and cardiac troponin I (cTnI) to estimate short term prognosis in dogs with heart failure caused by MVD. One hundred thirty nine dogs were distributed among five groups, according to ACVIM Stages. Groups B1, B2, C and D had a second blood sampling at day 60. Measurements were obtained for human Gal-3, NT-proBNP and cTnI. The researchers concluded that the magnitude and variation observed in Gal-3 did not allow for detection of differences between stages of MVD nor were capable of identifying patients in heart failure, compared to the other measured biomarkers, NT-proBNP and cTnI.
In a May 2018 abstract, Henry Ford Hospital researchers report finding that, in dogs in heart failure, increased levels of reactive interstitial fibrosis (RIF) is associated with increased interstitial levels of Gal-3.
In a July 2022 study, German researchers measured the effectiveness of Galectin-3, compared to N-terminal-prohormone-B-type natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI) -- to diagnosing the stage and progression of mitral valve disease (MVD) in 64 dogs (including 4 cavaliers) diagnosed by echocardiogram to be in various stages of MVD. They concluded that the biomarkers Galectin-3 and ST2 "indicate that they are unsuitable for diagnosing and staging canine DMVD patients", and that the diagnositic value of NT-proBNP and cTnI was confirmed.
RETURN TO TOP
---vitamin D
In an August 2015 study, a team of Japanese researchers examined vitamin D concentrations -- specifically serum 25-hydroxyvitamin D (25(OH)D) concentrations -- in 43 MVD-affected dogs, including 4 cavalier King Charles spaniels. They observed that serum 25(OH)D concentration begins to decrease before the onset of heart failure in MVD-affected dogs, and that vitamin D status is associated with the extent of heart enlargement. Previously, in a January 2014 study of serum vitamin D concentration in the bloodstreams of 82 dogs (31 with congestive heart failure [CHF] -- 20 with acquired valve disease (AVD) and 11 with dilated cardiomyopathy (DCM) -- and 51 unaffected control dogs, all over 5 years old), a team of US researchers found that low serum vitamin D concentration was associated with poor outcome in dogs with CHF. They suggested that strategies to improve vitamin D status in some dogs with CHF may prove beneficial without causing toxicity.
RETURN TO TOP
---serum cystatin-C (Cys-C) and symmetric dimethylarginine (SDMA)
Dimethylarginines (DMAs) are biological products derived by the methylation process of L-arginine. Symmetric dimethylarginine (SDMA) is used as a circulating biomarker of endothelial cell dysfunction, as is asymmetric dimethylarginine (ADMA). The surfaces of normal valve leaflets are lined with a layer of endothelial cells, aligned in uniform, parallel rows, making up the endothelium which covers a layer of collagen of the myocardium. "Endothelial cells" line the interior of blood vessels and are the layer that is in continuous contact with blood. The endothelium formed a complete and intact covering over the leaflets and chordae tendineae. Endothelial cells also line the interior of blood vessels and are the layer that is in continuous contact with blood and are a specialized category of epithelial cells.
In a September 2016 study by a team of Korean cardiology researchers, they examined serum concentration levels of two kidney function biomarkers -- Cystatin-C or cystatin 3 (Cys-C) and symmetric dimethylarginine (SDMA) -- which appear to play roles in predicting new-onset or deteriorating kidney diseases. Their goal was to evaluate these two renal disorder biomarkers in dogs with mitral valve disease (MVD) at varying degrees of heart failure. They found that serum Cys-C and SDMA concentrations were not correlated to age or body weight, but closely correlated to the severity of heart failure and echocardiographic markers of heart enlargement. The study found progressive elevation in renal markers in dogs with advancing MVD, suggesting that the reduction of glomerular filtration rate (GFR) might be worsened with the progression of cardiac dysfunction. They concluded that their study demonstrated that the glomerular filtration rate (GFR) -- a common complication in advanced stages of heart failure -- was decreased in dogs with MVD in earlier stages of heart failure, based on results from serum Cys-C and SDMA tests, and therefore, earlier intervention for preventing renal injuries even in asymptomatic MVD dogs is necessary for the successful long-term management of heart failure in MVD-dogs.
In an August 2018 article, Italian researchers studied 24 dogs, including 2 cavaliers, to evaluate SMDA in MVD-affected dogs in various stages (B1, B2, C, and D) to assess its reliability as a marker of early renal impairment in dogs affected by mitral valve disease. They report that:
"SDMA can be actually considered a reliable parameter for evaluation of renal function in dogs affected by MMVD, especially in those patients with a non-advanced stage of disease (ACVIM class B2), for which an early diagnosis of the onset of kidney failure is fundamental in order to plan a diuretic therapy. SDMA repeated measurements over time, as recommended by IRIS guidelines, are necessary, because one determination does not allow us to exclude definitely a later onset of the renal impairment and then to be considered diagnostic in order to highlight a possible onset of CRS [cardiorenal syndrome]."
In a September 2021 article, Italian researchers studied 85 MVD-affected dogs, including 9 (9.3%) cavaliers, to measure the plasma concentration of dimethylarginines in the various stages of MVD. They reported finding that "ADMA and SDMA were significantly associated with left atrium to aorta ratio, and creatinine, respectively. Increased plasmatic concentrations of dimethylarginines suggest a possible role as biomarkers of disease severity in dogs with decompensated MMVD."
RETURN TO TOP
---oxidative stress
In a June 2016 abstract, a team of Swedish and Denmark researchers compared levels of markers of oxidative stress in cavaliers with MVD, other MVD-affected breeds, and control group Beagles. They concluded that "Beagles and female dogs appear to have lower plasma concentrations of oxLDL [oxidized low-density lipoprotein]. The results cannot confirm a role of oxidative stress in dogs with MMVD."
---serum fatty acids
In a July 2016 article by a team of Japanese researchers, they studied 30 dogs with various stages of mitral valve disease, including seven cavaliers, to determine whether serum fatty acid concentrations in dogs with MVD differ from those in normal dogs (12 healthy dogs, including 1 CKCS). They found that several fatty acid concentrations were significantly lower in dogs with MVD, and that some of these concentrations and ratios correlated with echocardiographic parameters.
---serum leptin and adipokines
In an August 2016 article,, Korean researchers found that serum leptin concentrations were significantly higher in dogs with MVD than in healthy dogs, and that adiponectin concentrations were significantly lower in dogs with MVD.
RETURN TO TOP
---anemia
In an October 2016 article, a team of Taiwan researchers found in 114 MVD-affected dogs that the presence of anemia increased with the progression of MVD and was associated with the severity of heart failure. They stated that anemic dogs in heart failure had shorter median survival times (13 months) than non-anemic dogs in heart failure (28 months), and that anemia was a predictor of mortality.
---desmin, vimentin, periostin, caspase-3
In a December 2016 study by a team of Polish researchers, they examined the cell markers: (a) desmin, a standard cardiomyocyte marker; (b) vimentin, a typical cell marker expressed in mesenchymal cells; (c) periostin, a novel marker for myocardial remodelling; and (d) caspase-3, a marker of cell apoptosis. They compared them in dogs with end-stage dilated cardiomyopathy (DCM) and dogs with MVD and a control group of unaffected dogs. Their research focused upon the enlargement of the left atrium in the diseased dogs. They reported that changes included: (a) irregularity of desmin cross-striation and desmosomes; (b) a higher amount of vimentin-positive cells; (c) a change in the periostin expression pattern from cytoplasmic to extracellular; and (d) a lower expression of caspase-3. The alterations reportedly were more pronounced in the DCM group than in the MVD group. They concluded that, during heart failure, the pattern of desmin, vimentin, periostin and caspase-3 expression alters in the left atrium, regardless of the cause; that the changes are more pronounced in dogs with DCM than in dogs with MVD and similar left atrial enlargement.
---serum proteins
In a February 2017 article on a study of twelve cavaliers, an Italian team reported finding eight serum proteins which were differentially expressed between groups of moderately (M) and severely (S) affected CKCSs with MVD and a control group of healthy cavaliers. These proteins were found to be significantly related to the progression of the disease. In the M group versus healthy dogs complement factor H isoform 2, inhibitor of carbonic anhydrase, hemopexin, dystrobrevin beta isoform X7 and CD5 molecule-like resulted to be down-regulated, whereas fibronectin type-III domain-containing protein 3A isoform X4 was up-regulated. In the S dogs versus healthy group complement factor H isoform 2, calpain-3 isoform X2, dystrobrevin beta isoform X7, CD5 molecule-like and l-2-hydroxyglutarate dehydrogenase resulted to be down-regulated. Complement factor H isoform 2, calpain-3 isoform X2, dystrobrevin beta isoform X7, CD5 molecule-like and hydroxyglutarate dehydrogenase were found to be down-regulated in mild affected group versus healthy dogs. All of these proteins except complement factor H followed a decreasing trend according to the progression of the pathology. They concluded that the differential expression of serum proteins demonstrates the possibility these might be valuable for the detection and monitoring of the disease.
RETURN TO TOP
---serum homocysteine
In an April 2017 article, Korean investigators examined serum homocysteine in 63 dogs to determine if there is any correlation between homocysteine concentrations and the worsening of mitral valve disease. Homocysteine is an essential amino acid metabolite of methionine that is recycled into methionine or converted into cysteine by vitamin B. They found significant correlations between serum homocysteine concentrations and each stage of MVD (Stages B1, B2, C, D). They concluded that the measurement of serum homocysteine concentration may be useful in predicting the severity of MVD in dogs.
---serum nitrotyrosine
In an April 2017 article, Korean investigators examined serum nitrotyrosine in 50 dogs to determine if there is any correlation between nitrotyrosine concentrations and the worsening of mitral valve disease. Nitrotyrosine is an amimo acid product of tyrosine nitration which has been found to be a marker of cell damage and inflammation and appears to be undetected in healthy subjects. They found no significant correlation between serum nitrotyrosine concentrations in control dogs and dogs with mild MVD. However, dogs with moderate MVD had significantly higher serum concentrations of nitrotyrosine than that in the controls, and dogs with severe MVD had significantly lower serum concentrations of nitrotyrosine than that in moderate MVD dogs.
---heart-fatty acid binding protein (hFABP)
In a June 2017 abstract, Ontario Veterinary College researchers studied heart-fatty acid binding protein (hFABP) as a potential marker for MVD and dilated cardiomyopathy (DCM), compared to cardiac Troponin-I (cTnI) in affected dogs and healthy control dogs. They reported that levels showed significantly elevated hFABP with highest levels found in ACVIM Stage C disease regardless of cause, followed by Stage B and controls. cTnI differed in Stage C from both Stage B and controls, but was unable to differentiate between Stage B and controls. cTnI was indistinguishable between MVD-affected dogs and controls. They found that hFABP was the most significant predictor for death, and that the study showed the potential value of hFABP as a cardiac marker to differentiate stages of MVD in dogs.
---soluble ST2 (sST2)
In a June 2017 abstract, Brazilian researchers reported that concentrations of the soluble fraction of ST2 (sST2), a cardiac biomarker, higher than 40 pg/mL indicated higher mortality in dogs with advanced MVD.
In a July 2022 study, German researchers measured the effectiveness of ST2, compared to N-terminal-prohormone-B-type natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI) -- to diagnosing the stage and progression of mitral valve disease (MVD) in 64 dogs (including 4 cavaliers) diagnosed by echocardiogram to be in various stages of MVD. They concluded that the biomarkers Galectin-3 and ST2 "indicate that they are unsuitable for diagnosing and staging canine DMVD patients", and that the diagnositic value of NT-proBNP and cTnI was confirmed.
---adiponectin
Adiponectin is the most abundant plasma adipokine, and is well known for its role in energy homeostasis and cardiac protection. In a July 2017 article, researchers evaluated adiponectin serum concentrations and myocardial protein expression in MVD-affected dogs in CHF. They reported that serum adiponectin was significantly lower in dogs with CHF and lowest levels in the severest CHF class. Myocardial adiponectin was expressed independent of circulating adiponectin concentrations, suggesting a local regulatory mechanism within the heart.
---serum iron concentration
In an October 2018 article, a team of Italian veterinarians studied the case records of 54 dogs, measuring their serum iron concentration (SIC) and iron-capacity factors. They found that SIC was 18% overall, but that in MVD-dogs in Stage C (congestive heart failure) the SIC was 33%, and in dogs in Stage D (acute decompensated heart failure), the SIC was 100%. They did not find any significant differences in iron values between Stage B and Stage C dogs.
--- humanin (HN)
In a December 2018 article, a team of researchers at Chiang Mai University in Thailand report finding that in a study of 31 dogs (none were cavaliers) grouped in four classes (no MVD, Stage B, Stage C, and Stage D), the levels of humanin (HN), a polypeptide in blood plasma, was lower in dogs diagnosed with mitral valve disease (MVD), and that plasma HN were significantly lower in ACVIM Stages C and D than in healthy dogs. Also, they report finding that plasma NT-proBNP changed significantly only in MVD-affected dogs in Stage D.
RETURN TO TOP
-- quality of life questionnaire
The Functional Evaluation of Cardiac Health Questionnaire (FETCH) is a set of 18 questions for MVD-affected dog owners to answer. It has been developed based on widely accepted clinical signs of heart disease in dogs. (See box below.) The questionnaire consists of 18 questions to be answered by the dog's owner, who grades the severity of symptoms on a scale from 0 to 5, in which 0 = few symptoms and 5 = several symptoms, with higher scores indicating a poorer health-related quality of life. The questionnaire has been shown to be an effective, reliable method for assessing health-related quality of life in dogs with cardiac disease. See this June 2005 article. It also has been found to be valid as an independent measure of evaluating aproaching death due to MVD. See this March 2017 article.
FUNCTIONAL EVALUATION OF CARDIAC HEALTH QUESTIONNAIRE
These questions are included in the Functional Evaluation of Cardiac Health Questionnaire for dogs. Owners are asked to answer the following items to quantify their perception of the degree to which clinical signs of cardiac disease had affected the dog during the preceding 7 days. For each item, the owner grades severity on a scale from 0 to 5, where 0 = not at all, 1 = very little, and 5 = very much. Responses for each of the items are summed to obtain an overall score, with a possible total score ranging from 0 to 85. Because owners are instructed to skip 1 of 2 items (questions 5 and 6) depending on whether they had or had not been instructed to restrict their dogs' exercise, each owner provides responses for only 17 items.
How much did your dog's heart disease impact your dog's comfort or sociability during the last 7 days by:
1. making breathing difficult?
2. making your dog cough?
3.
making your dog wheeze when breathing?
4. making your dog generally
tired, fatigued or low on energy?
If the dog was on forced exercise restriction, the owner was instructed to skip question 5 and go to question 6. If the dog was not on forced exercise, the owner was instructed to answer question 5 and skip question 6.
5. making recreational pastimes (playing in the yard, playing fetch,
etc) difficult?
6. limiting your dog's favorite activities due to
exercise restriction?
7. making your dog sit or lie down to rest
during walks?
8. making walking around or climbing stairs difficult?
9. causing fainting or collapsing episodes?
10. making it difficult
for your dog to get comfortable?
11. making it difficult for your dog
to sleep through the night?
12. making your dog eat less than normal?
13. changing the types of foods your dog is willing to eat?
14.
increasing the number of urination accidents in the house?
15. making
your dog vomit?
16. limiting your dog's ability to spend time with
the family (can't get up stairs to join the family, can't get onto the
bed or couch, etc)?
17. making your dog irritable or unwilling to be
touched?
18. making your dog less bright and peppy?
(Source: Development and evaluation of a questionnaire for assessing health-related quality of life in dogs with cardiac disease. Lisa M. Freeman, John E. Rush, Andrew E. Farabaugh. J. Am. Vet. Med. Assn. June 2005;226(11):1864-1868.)
RETURN TO TOP
DNA Testing
Thus far, no specific gene mutation or even genetic region of the CKCS DNA has been confirmed as a cause for MVD in cavaliers. The occurrence of mitral valve murmur in the cavalir breed is believed to be due to a single major gene effect. Thus, breeding strategies to eliminate the disease cannot be based on genotype information at this time.
-- gene identification
 In
a 1996 study, Swedish researchers Lennart Swenson, Jens Haggstrom,
and Clarence Kvart, reported that MVD in cavaliers "is a polygenic
threshold trait and that sex of the offspring influences threshold
levels."
In
a 1996 study, Swedish researchers Lennart Swenson, Jens Haggstrom,
and Clarence Kvart, reported that MVD in cavaliers "is a polygenic
threshold trait and that sex of the offspring influences threshold
levels."
In a December 2022 article, Australian genetics researchers examined the genetic make up of 180 cavaliers with and without mitral valve disease (MVD) and in its various stages. They focused on mutations of the nebulette gene located at NEBL1, NEBL2, and NEBL3. Previous studies found that mutations in the nebulette gene appear to be associated with heart enlargement, possibly due to a poorer quality cytoskeletal framework of cardiomyocytes. These researchers compared 6 cavaliers being heterozygous (having two different alleles of the gene) for the protective/wild-type alleles of the NEBL gene, with the other 172 CKCSs which were homozygous for the NEBL1-3 alleles. They report finding that the 6 heterozygous dogs had smaller left atrium (LA:Ao) and left ventricle (LVIDdN) values than did the homozygous dogs. They concluded that:
"Since heterozygosity for the wild-type NEBL allele seems to reduce MMVD severity, CKCS breeders should plan to increase wild-type allele prevalence into the gene pool. There are two methods to accomplish this. The first is to apply genetic testing to identify registered CKCSs carrying the wild-type NEBL allele. Subsequently, breeders could be selecting for the wild-type allele, not against the derived allele, taking into consideration that selection for the wild-type allele should not reduce the gene pool too much. Second, breeders could introduce animals to the registry via an indexing scheme that enables the introduction of unregistered dogs (potentially of other breeds) carrying the wild-type NEBL allele into the registry system for this breed. While crossbreeding is a viable option for improving heart health in the CKCS, rules across many canine registries currently preclude this approach and such rules will likely take a long time to change. Selection for favourable dominant alleles present in the breed can be applied immediately. ... It is expected that the dogs heterozygous for wild-type alleles at these loci will experience reduced risk of mortality as a result of their lower LVIDdN and LA:Ao measurements."
The findings and conclusions in this December 2022 study appear to be drastically overstated. All 180 of the cavaliers in this study were Australian, making them quite insular, and only 6 of them (0.033%) were found to have the wild-type NEBL allele. Of those 6 cavaliers, 5 of them were diagnosed with MVD before the study began. So, only 1 out of 6 (16.6%) of these dogs did not have MVD at the time of the study.
Given that several prior studies (see below) have identitified other genetic regions as being associated with MVD in the CKCS, none of which have included the NEBL region, there is no reason to conclude from this December 2022 article that its investigators are on the right track. The authors even admit that:
"MMVD is complex in nature and unlikely to be controlled by a single gene. We expect that because of the CKCS breed history, some genes influencing this trait will have low variability. Other genes will be variable in the breed and lead to different individual risk levels within the CKCS population, with individual risk moderated by variants contributing to severity of disease."
Following this December 2022 study, the UK veterinary diagnostics testing laboratory Laboklin began offering a genetic test for cavalier King Charles spaniels, presumably for mutations of the nebulette gene (NEBL). Laboklin's test #8831 is charging US$76.80/UK£48.00 for each test. Laboklin falsely states "It is very unlikely that the dog will develop Myxomatous mitral valve disease (MMVD)." All published scientific evidence thus far expressly contradicts this assertion.
As noted above, thus far, there is no reliable evidence that NEBL mutations are a cause of MVD in cavaliers.
In a December 2024 article, genetic researchers examined the genetic makeup of 24 13+ year old cavalier King Charles spaniels, to determine which genes may be associated with longevity in the breed. All 24 13+ year old cavaliers and 17 younger "reference" CKCSs were owned by CKCS club members in the Czech Republic. Nine candidate genes were identified: B3GALNT1, NLRP1 like, PARP14 IQCJ-SCHIP1, COL9A1, COL19A1, SDHAF4, B3GAT2, and DIRC2. They concluded:
"The current knowledge shows that all the genes with SNPs [single-nucleotide polymorphisms] described in this study as associated with longevity in CKCS could directly or indirectly influence health and prolong life. We identified genes associated with common longevity-influencing factors such as cancer, immunity, DNA damage, cellular repair, and oxidative stress. We also found genes that may play a role in the tissue structure and formation. Most importantly, longevity-associated SNPs were found in genes that play a role in heart diseases, which are the major cause of death in the CKCS. We described SNPs in two collagen genes associated with longevity in the CKCS. Collagens were previously described to undergo morphological changes in the dogs affected with mitral valve disease, and changes in their expression in affected CKCS to have a direct effect on longevity. Our results show that the detected longevity-associated SNPs could also serve as potential markers of diseases."
--- history of gene research
In an April 2020 article, a University of Edinburgh team of veterinary researchers led by Dr. Brendan Corcoran (Greg R. Markby, Vicky E. Macrae, Kim M. Summers) examined the mitral valves of 30 euthanized MVD-affected dogs, including 8 cavalier King Charles spaniels (27%) to classify the valves according to MVD severity (Grade 0 - normal -- to Grade 4 - severely affected) and to identify all differentially expressed genes (DEGs) in all four such grades. By transcriptomic profiling they identified a total of 1,002 DEGs associated with a large number of gene families. Depending upon the grade of MVD, they found significant changes in gene expression intensity in especially ACTA2, HTR2B, MMP12, and CDKN2A. The TGFB1, TNF, IFGN genes were identified as the top up-stream regulators in the diseased valve samples. Of those, they found that TGFB1 appears to have the strongest associations with the disease. They concluded:
"In conclusion, despite differences in the total number of DEGs identified as canine MMVD pathology progresses over a lifetime, disease initiation, and progression appears to be primarily dependent on changes in TGFB signaling. This is the first description of the temporal and spatial expression of gene changes associated with naturally occurring myxomatous degeneration in any species. Other signaling pathways likely contribute to disease pathogenesis over time, with some becoming involved only at the stage of advanced disease. The factors that trigger the development of valve myxomatous degeneration are still unknown, but aberrant TGFB signaling appears to initiate and perpetuate the valve pathology characteristic of this disease in the dog."
Previously, different researchers had identified different chromosomes as possible regions for genes affecting mitral valve deterioration in MVD-affected dogs. The candidate regions include chromosomes CFA13, CFA14, 15, and 17, and genes COL1A2 (in CFA14) and CTNNA2 (in 17). See below for details on this research.
A team of veterinarians from Denmark, Sweden, Germany, England, and France reported in September 2011 that they have identified two specific locations on cavaliers' chromosomes CFA13 and CFA14 which are associated the breed's hereditary mitral valve disease. They grouped 139 cavaliers with early-onset MVD and 102 old CKCSs with no or mild signs of MVD as controls. Then they conducted a genome-wide association study to find specific locations associated with development of MVD. They also stated:
"We will initiate studies of the most promising candidate genes in the 2 candidate regions which hopefully will lead us to the mutations affecting the development of mitral valve disease."
However, in November 2011, a team of UK cardiologists and geneticists divided 36 CKCSs into groups of early and late onset MVD and assessed whether the distinction is determined by a small number of genetic factors. They report that they came up dry. They concluded:
"There were no regions of highly discrepant homo/heterozygosity in the two groups. Similarly, there was no evidence for loci associated with mitral valve murmur in a genome-wide association study. This analysis suggests that familial occurrence of mitral valve murmur in the CKCS breed is not due to a single major gene effect, indicating that breeding strategies to eliminate the disease cannot be based on genotype information at this time."
This seems to contradict the much more successful September 2011 report issued by the team of veterinarians from Denmark, Sweden, Germany, England, and France.
In an October 2015 study of Whippets, researchers found evidence that the severity of MVD degeneration is associated with chromosome 15 loci.
In a December 2016 report, a team of Columbian veterinary researchers investigated variations of the COL1A2 gene (within the CFA14 region) in 50 MVD-affected and 80 control Poodles. (The CFA14 region was identified in the September 2011 study, as being associated the CKCS's hereditary mitral valve disease. The Columbian veterinarians found that "the allele T of the rs22372411 variant was over-represented in MMVD patients compared with healthy controls." They concluded:
"Our results show for the first time an association of the rs22372411 COL1A2 gene variant with susceptibility to canine myxomatous mitral valve disease."
In a June 2017 abstract, Cornell University veterinary researchers conducted a genome-wide association study (GWAS) which revealed a strong association signal on Chromosome 17, near the CTNNA2 gene, which codes for alpha-catenin. They then identifying CTNNA2 as a candidate gene, which revealed a 53% decrease in normalized alpha-catenin levels and an 86% decrease in beta-catenin in MVD-affected dogs, when compared to control samples. However, no mutation of that gene was detected. The investigators surmised that a mutation may exist within the non-coding regions controlling CTNNA2 transcription, and they recommended further investigation.
In a December 2017 article, veterinary cardiologists examined the DNA in ten cavalier King Charles spaniels and ten Dachshunds affected with mitral valve disease (MVD), searching for varients of genes known to be analogous to genetic mutations associated with MVD in humans. They reported finding no such variants in any of the canine genes evaluated, and that they could not identify any genetic cause responsible for human MVD in the dogs studied. They concluded that MVD in the CKCS and Dachshund may not share an identical genetic cause with the human MVD.
In a March 2019 abstract, North Carolina State veterinary researchers report testing the involvement of serotonin in the development of mitral valve prolapse (MVP) in dogs. They obtained DNA samples from 51 dogs with MVP and performed whole genome sequencing. They searched for shared genes and/or gene families in the serotonin receptor signaling (SRS) pathway. They report that no single high impact variant or gene or gene family accounted for all of the dogs. They concluded that, although serotonin may play a role in the development of MVP, it may not be the primary genetic cause of the disorder.
In a December 2022 article, Australian genetics researchers examined the genetic make up of 180 cavaliers with and without mitral valve disease (MVD) and in its various stages. They focused on mutations of the nebulette gene located at NEBL1, NEBL2, and NEBL3. Previous studies found that mutations in the nebulette gene appear to be associated with heart enlargement, possibly due to a poorer quality cytoskeletal framework of cardiomyocytes. These researchers compared 6 cavaliers being heterozygous (having two different alleles of the gene) for the protective/wild-type alleles of the NEBL gene, with the other 172 CKCSs which were homozygous for the NEBL1-3 alleles. They report finding that the 6 heterozygous dogs had smaller left atrium (LA:Ao) and left ventricle (LVIDdN) values than did the homozygous dogs. They concluded that:
"Since heterozygosity for the wild-type NEBL allele seems to reduce MMVD severity, CKCS breeders should plan to increase wild-type allele prevalence into the gene pool. There are two methods to accomplish this. The first is to apply genetic testing to identify registered CKCSs carrying the wild-type NEBL allele. Subsequently, breeders could be selecting for the wild-type allele, not against the derived allele, taking into consideration that selection for the wild-type allele should not reduce the gene pool too much. Second, breeders could introduce animals to the registry via an indexing scheme that enables the introduction of unregistered dogs (potentially of other breeds) carrying the wild-type NEBL allele into the registry system for this breed. While crossbreeding is a viable option for improving heart health in the CKCS, rules across many canine registries currently preclude this approach and such rules will likely take a long time to change. Selection for favourable dominant alleles present in the breed can be applied immediately. ... It is expected that the dogs heterozygous for wild-type alleles at these loci will experience reduced risk of mortality as a result of their lower LVIDdN and LA:Ao measurements."
The findings and conclusions in this December 2022 study appear to be drastically overstated. All of the cavaliers in this study were Australian, and only 6 of them were found to have the wild-type NEBL allele. Given that several prior studies noted above have identitified other genetic regions as being associated with MVD in the CKCS, none of which have included the NEBL region, there is no reason to conclude from this December 2022 article that its investigators are on the right track. The authors even admit that:
"MMVD is complex in nature and unlikely to be controlled by a single gene. We expect that because of the CKCS breed history, some genes influencing this trait will have low variability. Other genes will be variable in the breed and lead to different individual risk levels within the CKCS population, with individual risk moderated by variants contributing to severity of disease."
In a January 2023 article, University of Copenhagen investigators report their examinations into the genetic makeup of 34 elderly (mostly aged 10 years) Swedish cavaliers, 19 of which had no mitral valve disease (MVD) or were in Stage B1 or B2, and 15 dogs in Stage C with severe MVD. Their aim was to identify genes which expressed themselves differently and which may explain the difference in the progression of MVD in the two groups of cavaliers. They identified 56 genes which were differentially expressed (DE) between the two groups. Of those 56 genes, several were either "up-regulated" or "down-regulated" in the group with no MVD or in its earlier stages, suggesting that these changes in those genes were an appropriate reaction to whatever was the cause of their MVD, and that those same genes in the dogs in the severe MVD group did not respond accordingly. Of those genes, up-regulation of particularly ALDH1A2 and RDH10 may be a compensatory reaction to MVD rather than a cause of disease. If so, then this up-regulation resulted in the disease not progressing into severe MVD with congestive heart failure (CHF). They also found three other genes, MYH1, LOC102724058, and CNTN3, with the greatest difference in expression between the two groups of cavaliers, and which were not part of an orchestrated response to disease but instead may be causative agents of MVD. They concluded:
"Based on these observations, we suggest that the primary cause of MMVD in CKCS may be found in a gene coding for one of the heart muscle proteins. However, the conclusion based on the present results is that an appropriate upregulation of MYH1 and downregulation of CNTN3, as a response to a so far unknown causative factor, can protect a dog with MMVD from developing CHF. An alternative conclusion is that an inappropriate downregulation and upregulation of MYH1 and CNTN3, respectively, in CKCS with MMVD may lead to development of CHF."
This 2023 Swedish report could be a very important step in the search for genetic causes of MVD in cavaliers, for a couple of reasons. First, the investigators acknowledge that differences in the way genes are expressed in cavaliers with and without severe MVD may be due to how the dogs compensate for the onslaught of MVD rather than being the cause of the MVD. Here, we find that two genes, ALDH1A2 and RDH10, are reactive in cavaliers with no or mild MVD and not so in Stage C dogs, and they do so in an orchestrated manner. Second, they identify three other genes, MYH1, LOC102724058, and CNTN3, which do not appear to be involved in compensating and therefore may be parts of the problem puzzle.
RETURN TO TOP
---microRNAs (miRNAs)
MicroRNAs (miRNAs) are regulators of gene expression in heart development and cardiac disorders. MicroRNAs are short, noncoding RNAs that are transcribed from the genome. The biological function of these small RNAs is to negatively regulate gene expression through mRNA degradation or translational inhibition. One microRNA may regulate several target mRNAs and have substantial effects on downstream gene expression networks. The human genome is believed to encode approximately 1,000 microRNAs, and most human protein-coding genes are regulated by these microRNAs. Circulating microRNAs can be detected in the blood, so that they may serve as circulating molecular biomarkers for various cardiac disorders.
In a June 2015 study, researchers examined the role circulating microRNAs may play as biomarkers of mitral valve disease. They examined 18 dogs, divided into three groups of six dogs each: (1) Stage A: normal dogs at risk of heart disease; Stage B1/B2: (2) asymptomatic dogs with MVD and mild to moderate cardiac enlargement; and (3) Stage C/D: dogs with MVD and CHF requiring multiple cardiac medications. They found that dogs in Stage B1/B2 or C/D had four upregulated miRNAs, including three cfa-let-7/cfa-miR-98 family members, while seven other miRNAs were downregulated, compared to Stage A. Expression of six of the 11 miRNAs also were significantly different between dogs in Stage C/D and those in Stage B1/B2. They observed that the expression changes were greater as disease severity increased. They concluded that their study suggests that there is an opportunity for using some circulating miRNAs as biomarkers for diagnosis, prognosis or monitoring response to treatment in MVD in dogs.
See also this July 2015 study by substantially the same researchers, in which they examined serum metabolites which they observed were significantly different between healthy and MVD dogs, which they concluded were representing alterations in fat and glucose energy metabolism, oxidative stress, and other pathways. They found that the three metabolites with the greatest single effect were γ-glutamylmethionine, oxidized glutathione, and asymmetric dimethylarginine, representing changes in energy metabolism, antioxidant function, nitric oxide signaling, and extracellular matrix homeostasis pathways. They opined that "Many of the identified alterations may benefit from nutritional or medical management. Our study provides evidence of the growing importance of integrative approaches in multi-omics research in veterinary and nutritional sciences." However, this study was funded by a major dog food manufacturer, and several of the researchers were employed by that manufacturer.
In a July 2017 article, a team of Tufts University investigators reported they had located microRNA (cfa-miR-9, cfa-miR-181c, cfa-miR-495 and cfa-miR-599) expressions which change with the progression of mitral valve disease and development of heart failure. The study consisted of 47 dogs, including 17 cavalier King Charles spaniels, which were divided into three groups based upon their heart conditions. All of the cavaliers in the study were MVD-affected and were in either the ACVIM Stage B (asymptomatic) or Stage C (heart failure) categories. The found:
"The present observations suggest that dysregulation of miR-9 and miR-599 chronologically precedes CHF, suggesting that these miRNA warrant further investigation as putative initiating factors for CHF, while others were found only at the time of CHF (miR-181c and miR-495), which denotes a role in initiation, progression or consequence of CHF. ... The data analysis shows that relative to young normal dogs, dogs with MMVD or dogs with MMVD-CHF, the expression level of cfa-miR-9 is low in older normal dogs. MiR-9 has been shown to have anti-fibrotic effects, and overexpression of miR-9 in neonatal rats inhibits the proliferation of cardiac fibroblasts and collagen production. ... Both of these effects would suggest that miR-9 has protective effects for cardiac remodelling."
"In contrast, cfa-miR-9 expression once again increases with the development of MMVD, which would suggest that MR [mitral regurgitation], volume-overload, cardiac remodelling or haemodynamic effects on other organs (lungs and kidneys) may initiate or propagate higher expression of circulating cfa-miR-9. However, it is not known if the anti-fibrotic effects of miR-9 are sufficient to counter pro-fibrotic stimuli exerted by volume overload and chronic heart failure."
"The data showed that there is high expression of exosomal cfa-miR-181c in dogs with CHF. MiR-181c is encoded in the nucleus and assembled in the cytoplasm of the cardiomyocytes and is then translocated into mitochondria to regulate mitochondrial gene expression. ... [G]iven the significant increase in cfa-miR-181c expression in MMVD-CHF, miR-181c may serve as an important biomarker or therapeutic target in patients at risk for developing CHF."
"Plasma exosomal miR-495, which is repressed with age, increases with MMVD-CHF. This ex-miRNA was upregulated with heart disease, particularly in advanced disease with CHF in defiance of its age-related trend downward. ... Although speculative, cfa-miR-495 may also serve as a potential biomarker to help monitor progression to active CHF in addition to cfa-miR-181c."
"Exosomal miR-599 declines in MMVD compared to age-matched controls. ... An increase in cfa-miR-599 associated with ageing was observed. Studies have shown that this miRNA can inhibit vascular smooth-muscle cell proliferation and production of collagen I, collagen V and proteoglycan by direct targeting of TGFB2. Interestingly, there is a downregulation of cfa-miR-599 in dogs with MMVD when compared to normal older dogs."
"Based on the present disease model, two miRNA were found, specifically miR-495 and miR-181c, whose rise in expression level coincides with the observation of CHF. The authors acknowledge that dysregulation of these ex-miRNA may not be unique to CHF secondary to MMVD, but rather may be a signature of canine heart failure which is a consequence of other aetiologies."
In a March 2022 article, Italian researchers compared the levels of the microRNA miR-30b-5p in 44 cavalier King Charles spaniels, which were classified into four groups of 11 dogs each: Stage A, Stage B1 under age 3 years, Stage b1 between ages 3 and 7 years, and Stage B1 over age 7 years. MVD was diagnosed by echocardiographic examinations, meaning that Stage A cavaliers had no echocardiographic evidence of mitral regurgitation and Stage B1 dogs had any evidence of thickening mitral valves and regurgitation. The investigators report finding that in each group of Stage B1 dogs, the expression levels of miR-30b-5p was significantly higher than in the healthy Stage A cavaliers. They concluded:
"This study identified a biomarker that may have an impact in both implementing preventing programs through genetic selection and in clinical practice. These results confirm that in CKCSs, as already demonstrated in humans, there is a differential expression of miRNAs, suggesting that their expression profiles are distinct for dogs with MMVD compared to healthy dogs. We demonstrated that miR-30b-5p could discriminate among ACVIM stage A CKCSs and ACVIM stage B1 CKCSs younger than 3 years, without heart murmurs, without clinical signs, but with an echocardiographic diagnosis of MMVD. ... The present results lay the basis for a breeding program that will help the CKCS' breeders in their targeted selection to obtain healthier subjects with a reasonable life expectancy. At the same time, highlighting the risk of the development of the disease at an earlier stage will favour a preventive screening and a mitigating therapeutical approach."
In a September 2022 article, the same Italian veterinary researchers report comparing a microRNA biomarker with echocardiographic measurements of cavaliers diagnosed with various degrees of MVD. The investigators focused on miR-30b-5p and compared its expression with clinical and echocardiographic values of 35 CKCSs at different stages of MVD. Their stated goal was to investigate reliable early miRNA biomarkers of MVD, to see if they could offer additional advantages in diagnosing the disease early in its progression and try to improve the timing of treatment and life expectancy. They categorized the MVD-affected cavaliers in Stages A, B1, B2, and C according to the 2019 ACVIM Consensus Statement definitions. They report finding that high levels of plasma miR-30b-5p corresponded to lower LVIDdN (left ventricle dimension value), ESVI (left ventricular end-systolic volume index), EDVI (left ventricular end-diastolic volume index), and LA/Ao (left atrial dimension value), all being echocardiographic values. They concluded that their study allows the identification of a relationship between high levels of miR-30b-5p and forms of MVD which appear echocardiographically more stable over time in the CKCS.
In a February 2026 article, the same team of Italian researchers profiled salivary microRNAs (miRNAs) of 25 cavaliers -- 6 in ACVIM Stage A, 13 in Stage B1, and 6 in Stage B2. Their aim was to identify the target genes. They report finding that 25 miRNAs were differentially expressed (DE) between Stages A and B1 dogs; 35 DE-miRNAs were modulated in Stage B2 compared to the Stage B1 cavaliers; 4 miRNAs were up-regulated in Stage B1 CKCSs compared to both Stages A and B2. They concluded that their findings confirm that salivary miRNAs can be successfully quantified in CKCSs and that their expression differs across MVD severity groups.
RETURN TO TOP
-- cell transitions
- senescent cells
- TGF-B1
- autophagy
- potential medications to reverse senescent cells
- FGF21 (fibroblast growth factor 21)
Deterioration of the mitral valve, which is how MVD "progresses" over time, has been found to be caused by destruction and disorganisation of the valve's extracellular matrix (ECM) (the connective tissue between the cells of the valve), as a result of (a) myxomatous degeneration -- an abnormal buildup of mucopolysaccharides in those connective tissues, and (b) the valve's interstitial cells transitioning from a normal quiescent (qVIC) phenotype to an activated myofibroblast (aVIC) phenotype. These aVICs have been found to be persistent in diseased mitral valves, and unlike typical aVICs, these in the mitral valves persist and do not eventually reach the end of a finite life-span and die. They are called senescent cells. The mechanisms which initiate and progress MVD development are due to changes in the TGFB signaling pathway.
 In a
February 2004 study, a team of UK researchers led by Dr. Brendan M.
Corcoran (right), observed the mitral valves of dogs with deteriorated mitral
valves due to MVD. They noticed (a) tissue swelling on the edges of the
valve leaflets, chordae tendineae, and the chordal-papillary muscle
junction; and (b) the denudation of endothelial cells, primarily along
the leaflet edges, exposing the basement membrane or subendothelial
valve collagen matrix.
In a
February 2004 study, a team of UK researchers led by Dr. Brendan M.
Corcoran (right), observed the mitral valves of dogs with deteriorated mitral
valves due to MVD. They noticed (a) tissue swelling on the edges of the
valve leaflets, chordae tendineae, and the chordal-papillary muscle
junction; and (b) the denudation of endothelial cells, primarily along
the leaflet edges, exposing the basement membrane or subendothelial
valve collagen matrix.
The surfaces of normal valve leaflets are lined with a layer of endothelial cells, aligned in uniform, parallel rows, making up the endothelium which covers a layer of collagen of the myocardium. "Endothelial cells" line the interior of blood vessels and are the layer that is in continuous contact with blood. The normal dogs' mitral valve's endothelial cells appeared orderly in a set pattern that corresponded with the orientation of underlying bundles of collagen. The endothelium formed a complete and intact covering over the leaflets and chordae tendineae.
At the time of that 2004 study, the researchers stated that whether the observed endothelial loss is a consequence or cause of the MVD process remains to be seen. Since then, much has been learned.
In a March 2015 study, Dr. Corcoran and his research team examined 5,397 differentially expressed canine genes, narrowing down their examination to 591 genes in six to ten biological function clusters -- relevant to inflammation, cell movement, cardiovascular development, extracellular matrix organisation and epithelial-to-mesenchymal (EMT) transition in dogs with mitral valve disease (MVD). They stated:
"Considering the biological relevance to MMVD, the gene families of importance with significant difference between groups included collagens, ADAMTS peptidases, proteoglycans, matrix metalloproteinases (MMPs) and their inhibitors, basement membrane components, cathepsin S, integrins, tight junction cell adhesion proteins, cadherins, other matrix-associated proteins, and members of the serotonin (5-HT)/transforming growth factor -B signaling pathway."
In an August 2015 study published by Dr. Corcoran and the same team of researchers, they examined the mitral valve leaflets of 14 deceased cavaliers which had suffered from MVD, and compared them with the leaflets of 5 unaffected mixed breed dogs. The aim of their study was to investigate the possibility of re-activation -- meaning post-embryotic -- and recruitment of embryotic developmental processes MVD, such as the transition of epithelial cells and endothelial cells to mesenchymal cells.
"Epithelial cells" line the cavities of tissues throughout the body and cover flat surfaces and form glands. "Endothelial cells" line the interior of blood vessels and are the layer that is in continuous contact with blood and are a specialized category of epithelial cells. "Mesenchymal cells" develop into any types of connective or supporting tissues, smooth muscle, vascular endothelium, and blood cells. "EMT" is a process by which epithelial cells lose their cell polarity and cell-cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells. Similarly, "EndoMT" is the same process for the transition of endothelial cells to mesenchymal cells. Previous research has shown that EMT and EndoMT contribute to a range of chronic degenerative diseases and cancer metastasis.
The Corcoran team found significant differential expression for genes typically associated with EndoMT -- including markers of inflammation (IL6, IL18 and TLR4), basement membrane disarray (NID1, LAMA2 and CTSS), mesenchymal and endothelial cell differentiation (MYH11 and TAGLN) and EndoMT (ACTA2, SNAI1, CTNNB1, HAS2, CDH5, and NOTCH1). In heart valves of MVD-affected dogs, there was increased expression of these genes, with the exception of NOTCH1, and a reduction in CDH5. Apart from down-regulation of NOTCH1 expression, all other changes support a potential contribution of EndoMT and endothelial migration in the development of MVD.
The research findings confirm the study's aim and "strongly suggest involvement of developmental signaling pathways and mechanisms, including EndoMT, in the pathogenesis of canine MMVD." Most significantly, the findings suggest that, instead of MVD being a degenerative condition, MVD may be due to the development of EMT and EndoMT. The researchers point out that this is the first report of involvement of development signaling pathways and endothelial to mesenchymal transition in canine MVD. They also provide information on biological mechanisms that have therapeutic potential for new drug discovery.
In a September 2015 master's thesis by a Colorado State Univ. student, she similarly concluded that "active EndMT in canine degenerative mitral valves could be contributing to the formation of high cellular density myofibroblast transformation which has been postulated to mediate mitral valve degeneration."
Normal cells which make up the tissues in the dog's body have a life
cycle, which consists of both growth and
 division. The cell grows by
accumulating nutrients. While some healthy cells do not progress to the
point of dividing or remain in a non-dividing state for a long time (quiescent
cells), others go through a process of division (mitosis),
which includes duplicating its DNA and its other contents (e.g.,
organelles, chromosomes) and creating two "daughter" cells, after which
the "parent" cell ceases to exist. Normal cells also are programmed to
die (apoptosis) when no longer needed or when they become a threat to
survival.
division. The cell grows by
accumulating nutrients. While some healthy cells do not progress to the
point of dividing or remain in a non-dividing state for a long time (quiescent
cells), others go through a process of division (mitosis),
which includes duplicating its DNA and its other contents (e.g.,
organelles, chromosomes) and creating two "daughter" cells, after which
the "parent" cell ceases to exist. Normal cells also are programmed to
die (apoptosis) when no longer needed or when they become a threat to
survival.
"Senescent cells" is the name given to cells in the dog's body which, unlike normal cells, fail to continue to divide amd yet also fail to die. The cause of senescense may be due to a reponse to a variety of external stress, DNA damage, or other internal factors. These senescent cells also may wreak havoc to the tissues surrounding them by remaining active and releasing damaging proteins which can cause other cells to become senescent.
In the normal canine mitral valve there exists valve interstitial cells (VICs), the most common form of cell in all three layers of the dog's mitral valve, which are specialized quiescent cells (qVICs) which become activated (aVICs) in response to damage to the mitral valve. When activated, these aVICs excrete collagen and other connective tissue substances as needed and then return to a quiescent state or are removed from the valve by programmed cell death (apoptosis). See this August 2024 article.
In cavaliers affected by MVD, the Corcoran team has found that aVICs fail to die and instead remain activated to become senescent cells, thereby damaging neighboring cells. The Corcoran team thus is focused upon finding medical treatments for controlling the creation of these senescent cells in cavaliers' mitral valves.
TGF-B1 (transforming growth factor beta 1):
Transforming growth factor beta (TGF-B) is a combination of growth factors which are secreted to support cellular growth in dogs and most all other mammals. The progression of MVD has been found to involve a transition of normal valve cells (qVICs) to an activated myofibroblast type of cell (aVICs), and this transition is dependent on abnormal TGF-B signaling. The mechanisms which initiate and progress MVD development have been found to be due to changes in the TGF-B signaling pathway. In this May 2022 article, Dr. Corcoran thoroughly summarizes the current knowledge about the role of TGF-B in the onset and progression of MVD in dogs.
Transforming growth factor beta 1 (TGF-B1) is a polypeptide in the TGF-B family of cytokines. It is a secreted protein that performs certain cellular functions, including control of cell growth, cell proliferation, cell differentiation, and apoptosis.
In an August 2008 article, German researchers examined the role of TGF-B1 and reported that in the heart, TGF-B1 is one of the main factors that cause cardiac fibrosis. In that study of the hearts of 40 deceased dogs, consisting of 10 normal hearts, 7 hearts with mild MVD, and 23 hearts with moderate or severe MVD, they found that TGF-B1 expression increased markedly as MVD progressed.
In an August 2019 article, Dr. Corcoran has found that, using mitral valve cells from healthy and MVD-affected dogs, they were able to model heart cell transitions due to MVD by using TGFB1 to transform normal mitral valve interstitial cells (qVICs) into MVD-diseased activated myofibroblasts (aVICs) and then reverse that transformation by using pan-anti-TGFB antibodies and the kinase inhibitor SB431542. They also were able to perform that reverse transformation when starting with diseased cells. TGF-B1 is one of a set of multifunctional peptides that controls proliferation, differentiation, and other functions in cells. The researchers noted that:
"This is the first time that an effective reversal of induced- and naturally-occurring disease phenotype has been achieved for MMVD, albeit under cell culture conditions, and has implications for understanding pathogenesis and discovery of novel therapeutic options applicable to MMVD."
In November 2019, researchers at Tufts' Cummings veterinary school announced an intention to conduct a clinical study in which MVD-affected cavaliers in Stage B2 (murmur and enlargement but not CHF) will be injected with an AAV medium which will contain "transforming growth factor beta receptor 2 (sTGFBR2)", a repressor of TGF-B1, in that it inhibits the action of TGF-B1, most likely by adsorbing TGFB1 or by acting as a dominant negative receptor.
In a February 2020 article, Dr. Corcoran's team furthered their study of deteriorating cells in MVD-affected cavaliers. They hypothesised that there is survival of activated myofibroblasts in MVD-affected valves and that this, in part, can be explained by a defect in the activation and progression of apoptotic pathways. They examined the distribution of activated myofibroblasts (valve interstitial cells -- aVICs) to confirm their survival, and then they examined the expression of apoptotic genes by qVICs and aVICs in cell culture, before and after stimulation with doxorubicin, a chemotherapy drug which works by slowing or stopping the growth of certain types of cells. Their data indicated that the ability of aVICs to survive in mitral valves may be due to a heighthened resistence to apoptosis (the programmed death of most cells after a specified life-span).
In an April 2020 article, Dr. Corcoran's team (Greg R. Markby, Vicky E. Macrae, Kim M. Summers) examined the mitral valves of 30 euthanized MVD-affected dogs, including 8 cavalier King Charles spaniels (27%) to classify the valves according to MVD severity (Grade 0 - normal -- to Grade 4 - severely affected) and to identify all differentially expressed genes (DEGs) in all four such grades. By transcriptomic profiling they identified a total of 1,002 DEGs associated with a large number of gene families. Depending upon the grade of MVD, they found significant changes in gene expression intensity in especially ACTA2, HTR2B, MMP12, and CDKN2A. The TGFB1, TNF, IFGN genes were identified as the top up-stream regulators in the diseased valve samples. Of those, they found that TGFB1 appears to have the strongest associations with the disease. They concluded:
"In conclusion, despite differences in the total number of DEGs identified as canine MMVD pathology progresses over a lifetime, disease initiation, and progression appears to be primarily dependent on changes in TGFB signaling. This is the first description of the temporal and spatial expression of gene changes associated with naturally occurring myxomatous degeneration in any species. Other signaling pathways likely contribute to disease pathogenesis over time, with some becoming involved only at the stage of advanced disease. The factors that trigger the development of valve myxomatous degeneration are still unknown, but aberrant TGFB signaling appears to initiate and perpetuate the valve pathology characteristic of this disease in the dog."
In his May 2022 article summarizing the role of TGF-B in the onset and progression of MVD, Dr. Corcoran concludes:
"Phenotypic transition of qVICs to aVICs induced by dysregulated TGF-B signaling appears to be a key contributor to valve myxomatous degeneration though aberrant matrix remodeling, exerting control through complex canonical and non-canonical signaling pathways interactions and effects, which can conceivably affect the disease phenotype alone or in combinations. To what extent one of these might be a dominant pathway for the diseased is still unknown. Furthermore, what abnormal signaling contributes to the survival and persistence of aVICs in diseased valves remains unanswered. Understanding the mechanisms that control cell persistence in this disease likely will give clues to the pathogenesis and identify potential therapeutic targets in both the dog and human."
Autophagy is a process in the healthy dog's body (as well as all mammals) that rapidly degrades and recycles components of damaged cells, such as the aVIC senescent cells described above. Autophagy normally plays a crucial role in maintaining cellular health by removing aged cells at the end of their life cycle. Autophagy consists of numerous receptors which are selective in choosing which cell components shall be degraded and recycled, including cyclin-dependent kinase inhibitor (CDKI) proteins.
In this February 2025 article, the Corcoran team reports finding that the senescent cells derived from dogs diagnosed with early (Stage B1) to mid-stage (Stage B2) MVD display deficient autophagy performance in degrading and recycling the components of those senescent cells. Specifically, they find that the autophagy receptor SQSTM1 is specific for degrading CDKI proteins CDKN2A and CDKN1A. In dogs with MVD, it appears that autophagy's receptor SQSTM1 is somehow made inoperable ("silenced"), thereby failing to degrade CDKN2A and CDKN1A and allowing their inceased accumulation. This February 2025 study indicates that the impairment of autophagy plays a role in inducing qVICs to become senescent cells. See also this April 2025 abstract of the February 2025 article.
In the February 2025 study, the investigators also found that cells treated with the drug rapamycin, an MTOR inhibitor, reversed cell senescence and promoted autophagy of CDKN2A and CDKN1A on a short-term basis. However, they also found that prolonged exposure to rapamycin had the opposite effect.
Potential medications to reverse senescent cells:
At this stage of cellular research by the Corcoran team members, they work with individual cells removed from the dogs' mitral valve tissues. It essentially is Petri dish research, more properly called in vitro cultures. They analyze how the cells should be expected to respond to various potential treatments. Thus far, a couple of known treatments have been mentioned -- quercetin and rapamycin. But none are ready for prime time. In vitro first must lead to in vivo -- how the cells in the living bodies of the dogs react.
In a March 2023 article, Dr. Corcoran and his team report finding some very important relationships between cellular transmissions and the deterioration of the mitral valve in MVD-affected dogs. Their findings indicate potential medical remedies to delay the progression of MVD and possibly even reverse that process.
Their research focuses on the "valve interstitial cells" (VICs), the most common form of cell in all three layers of the dog's mitral valve. Healthy VICs are called "quiescent" VICs (qVICs). The transforming growth factor beta (TGF-B) is a category of proteins involved in communicating with various cells, including VICs, and has been shown to convert healthy qVICs into abnormal, diseased "activated myofibroblast" versions (aVICs), by sending specific signals through a "pathway"consisting of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), which regulates cell differentiation. This PI3K/AKT/mTOR/p70 S6K pathway has been identified as contributing to several cardiovascular disorders, and in our case, converting healthy qVICs to diseased myofibroblast versions, aVICs, in MVD-affected dogs.
In this March 2023 study, the mitral valves of six diseased dogs, including a cavalier, and six healthy dogs were examined. The diseased dogs' valves included aVICs and the healthy dogs' valves included qVICs. The researchers found in the diseased aVICs high levels of TGF-B secreted into the mitral valves and activation of the PI3K/AKT/mTOR pathway. Once the VICs were converted from qVICs to aVICs, they were programmed to not expire, so they continued to exist in their abnormal state and continued to secrete a complex mixture of factors causing continual cell matrix disorganization and alter the behavior of nearby otherwise healthy cells.
The researchers hypothesized that one or more medications, called antagonists, could inhibit the damaging signaling caused by TGF-B and PI3K/AKT/mTOR/p70 S6K, which converts the VICs from healthy to damaged. They called this "pharmacological inhibition". They selected a combination of drugs - LY294002 (an inhibitor of PI3K), copanlisib (Aliqopa, BAY-80-6946 - also a PI3K inhibitor), and alpelisib (Piqray - another PI3K inhibitor). These three served to inhibit the PI3K signaling and restored the healthy qVICs and suppressed the damaging effects of the aVICs. They emphasize that all of this research has been performed outside of a real dog. They point of that:
"The findings of this study will require in vivo validation. Although in vitro primary cells isolated from clinical samples are largely able to reflect the disease parthenogenesis, in vivo validations are needed considering the drug metabolism, individual variance and clinical transitional research. However, there are a limited numbers of animal models available to study the pathogenesis of MMVD."
They hypothesized that quercetin, a flavonoid found in fruits and vegetables, such as blueberries, kale, and broccoli, may remove senescent cells in the same manner as LY294002, copanlisib, and alpelisib used in their study. However, they suggest that the effect of quercetin "may cause unexpected valvular outcomes and it is likely reversing aVICs to a non-senescent qVICs ... might be a more promising therapeutic approach to control MMVD in both the human and the dog." They conclude:
"In conclusion, TGF-B-induced PI3K/AKT/mTOR/p70 S6K signaling controls the phenotypic transformation and functions of VICs in canine MMVD. Pharmacological inhibition of PI3K signaling reverses diseased senescent VICs, with improved capacity for apoptosis and autophagy. Furthermore, downstream mTOR/p70 S6K signaling plays an important role in the regulation of VIC transformation, ECM protein synthesis, apoptosis, autophagy and senescence in MMVD. This work informs the naturally occurring disease in dogs as a novel large animal model to investigate human early stage MMVD and warrants further investigations of senolytic compounds or autophagy activators as a potential novel therapeutic strategy for the treatment of MMVD and other age-related degenerative disorders."
In an October 2023 article, Dr.. Corcoran's team examined the mitral valves of 12 deceased dogs -- 6 with normal hearts and 6 with MVD-diseased hearts, including one cavalier -- to identify changes in downstream signals in the TGFB pathway in canine mitral valve disease (MVD) and examine the effects of antagonism of one significant signal, SMAD2. SMAD are a family of proteins that are the main signal transducers for receptors of the transforming growth factor beta (TGFB) superfamily, which plays a vital role in regulating cell development and growth. SMAD2 is a protein that is encoded by the SMAD2 gene. SMAD2 mediates the signal of TGFB and regulates certain cellular-damaging processes, including converting normal quiescent valve interstitial cells (qVICs) of the mitral valve into disease-derived activated myofibroblasts (aVICs). SMAD2 protein expression can be regulated ("antagonized") by inserting certain other proteins which in this case were able to transition diseased aVICs cells back to the normal phenotype qVICs. They also found similar characteristics in another TGFB pathway, identified as PI3K/AKT/mTOR. The team's ultimate goal is to develop new therapies for treating MVD in dogs by preventing normal mitral valve cells from being converted to diseased cells and also by reverting diseased cells back to normal ones.
In this August 2024 article, Dr. Corcoran expounded upon his team's research into treating senescent aVIC cells. He observed that the flavonoids quercetin and fisetin target different points of the senescent signaling pathway. Quercetin inhibits the PI3K-AKT pathway, and fisetin is a topoisomerase inhibitor. Also, the prescription drug dasatinib (Sprycel) is a tyrosine kinase inhibitor and has been shown to improve flavonoid effects. He wrote:
"Our study showed that all three can inhibit senescence in aVICs, but that quercetin is more potent than fisetin, and the effect is more pronounced when quercetin and dasatinib are used together. While this was only examined in cell culture, it is a proof of principle that MMVD-associated cell senescence can be countered, and these findings will inform future studies in clinically affected patients."
In this February 2025 study, the investigators found that VIC senescent cells treated with the drug rapamycin, an MTOR inhibitor, reversed cell senescence and promoted autophagy of CDKN2A and CDKN1A on a short-term basis. However, they also found that prolonged exposure to rapamycin had the opposite effect.
FGF21 (fibroblast growth factor 21):
Fibroblast growth factor 21 (FGF21) is a secreted protein in a class of polypeptide growth factors, that has been found to act as a metabolic regulator and protect cardiac cells against enlargement in mice when injected into them using an adeno-associated virus (AAV) as the transmitting medium. In November 2019, researchers at Tufts' Cummings veterinary school announced an intention to conduct a clinical study in which MVD-affected cavaliers in Stage B2 (murmur and enlargement but not CHF) will be injected with an AAV medium which will contain FGF21.
RETURN TO TOP
Inflammation
Several inflammatory substances, such as C-reactive protein (CRP), endothelin (ET), interleukins (IL), and tumor necrosis factor alpha (TNF-a) have been found to play roles in the progression of mitral valve disease leading to heart failure in dogs.
C-reactive protein (CRP) is released from hepatic cells after stimulation by cytokines early in the course of the inflammatory process. Increased serum values of CRP have been observed in dogs with MVD in some circumstances. See our C-reactive protein discussion in our Diagnosis section above.
Cytokines are a category of proteins which are secreted by cells and have a specific effects upon their own cell and interactions with other cells as they circulate through the blood system. There are inflammatory cytokines and anti-inflammatory cytokines. Interleukins (IL), including interleukin-1B (IL-1B), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-10 (IL-10) are inflammatory cytokines.
In a 2014 Italian doctoral thesis, the researcher suggested that MVD appears to be associated with a chronic state of inflammation, as evidenced by measurements of immunoglobulin antibodies and glycoprotein and complement proteins particularly associated with immune responses to inflammation.
In a March 2016 article, researchers examined the levels of cytokine expression in peripheral blood mononuclear cells (PBMC) of dogs with MVD (none were cavaliers) at different stages and in healthy dogs. They investigated potential relationships between cytokine expression and echocardiographic indices of MVD severity, left ventricular (LV) function and remodelling. For this purpose, IL-102 1, IL-6, IL-8, TGF-B1 and TNF-a peripheral blood mononuclear cells (PBMC) expression was measured. In the dogs with MVD, there were differences in IL-8 and TGF-B1 mRNA concentrations between the ACVIM stages of MVD (Stages B1, B2, and C). No statistically significant differences were detected between groups for IL-1a, IL-1B, IL-6, or TNF-a PBMC expression. IL-8 expression increased with increasing MVD severity and TGF-B1 expression was higher in asymptomatic dogs with echocardiographic signs of heart enlargement (Stage B2) than in all other groups. They concluded that their results could suggest the involvement of these cytokines at different stages of MVD.
In a December 2017 article, Brazilian researchers investigated the concentration of IL-1B, 4, 6, 10, TNF-a and CRP in MVD-affected dogs of ACVIM Stages B1, B2, and C, along with a control group of healthy dogs. The report finding: (1) the serum levels of interleukin 1B increases in MVD-affected dogs, and even higher levels are observed in dogs with MVD symptoms; (2) there is a significant correlation between heart enlargement and congestive heart failure and the circulating levels of IL-1B and IL-4; and (3) the serum levels of IL-6 and the left-ventricular shortening fraction are negatively correlated.
In a June 2022 article, Italian researchers investigated changes in blood pro-inflammatory cytokines and immunophenotypes in 36 dogs affected by mitral valve disease at different stages (Stages B1, B2, C, D). Seven of the MVD-affected dogs were cavaliers (19.5%), the most of all purebreds in the study. Their aim was to verify the existence of a pro-inflammatory condition and a dysfunction of the immune system in MVD-affected dogs in different stages of severity through the blood assessment of CD4+FoxP3+ regulatory T-cells (Treg) cells, leptin, and three pro-inflammatory cytokines (tumor necrosis factor [TNF]-a, interleukin (IL)-1B, and IL-6 pro-inflammatory cytokines). They claim this to be the first study to investigate changes in blood pro-inflammatory cytokines and immunophenotypes in dogs based upon their stages of MVD. They concluded:
"Overall, our results show a significant increase in cytokine levels in dogs with more advanced MMVD and therefore with greater hemodynamic impairment, and the relative increase in Treg cells could represent a regulatory mechanism that limits the inflammatory immune response. Furthermore, the positive correlation between IL-6 and the LV diastolic volume suggests that inflammatory activation may play a role in the ventricular remodeling associated with the progressive volumetric overload in MMVD."
Leaky Gut Syndrome: There is a theory being examined of an inflammatory connection between the microbiotic contents of the dogs' intestines -- it's gut -- and the progression of MVD. The gut holds trillions of bacteria and other microorganisms of several varieties, all of which must be maintained in balance to keep the dog healthy. When these microbiota become imbalanced, a process called "gut dysbiosis" occurs, combined with inflammation of the membrane which lines the intestines -- the gut barrier -- causing "intestinal permeability" or "leaky gut". The gut barrier is designed to allow specified molecules to pass through, but when leaky gut syndrome occurs, the passage way becomes stuck open, resulting in the spread of inflammation to other organs of the body, especially the heart. Thus far, no definite differences have been found in veterinary publications between the bacteria in the gut and spreading through the bloodstream and the stages of MVD (B1, B2, C/D).
In a November 2025 article, USA veterinary researchers explored the extent of the relationship between dogs diagnosed with MVD and leaky gut (what they called "gastrointestinal bacterial translocation"). More specifically, they focused upon cardiac inflammation in MVD-affected dogs. Only 25 dogs diagnosed with MVD, including only 2 cavaliers, were involved in this study. Serum lipopolysaccharide (LPS), interleukins (i.e., IL-2, IL-6, IL-8), tumor necrosis factor-alpha, and cardiac troponin were measured over the course of the study The MVD-dogs were divided into Group 1 (Stage B1 dogs) and Group 2 (Stages B2 and C dogs). All of these dogs were untreated for MVD. They found that 20% of the dogs in Group 1 had GI clinical signs, compared to 66.7% of those in Group 2, and that serum IL-6 and LPS concentrations were significantly associated with MMVD stage severity. They concluded that:
"This study indicated that dogs with MMVD have evidence of loss of gastrointestinal barrier function as evidenced by bacterial translocation as the disease progresses in severity, which may be associated with systemic inflammation. ... We also observed an association between disease severity and both endotoxemia and systemic inflammation, a new observation in dogs with MMVD. ... These findings warrant further evaluation of gastrointestinal barrier function and maybe even the gastrointestinal microbiome as therapeutic targets in dogs with MMVD."
RETURN TO TOP
Stages of MVD
- NYHA Classes of Heart Disease
- ISACHC Classes of Heart Disease
- 2009 ACVIM Consensus Statement
- 2019 ACVIM Consensus Statement
- Mitral INsufficiency Echocardiographic score (MINE)
As mitral valve disease (MVD) worsens, cardiologists have categorized the disease in stages. Initially, they used the human heart disease classification schemes devised by the New York Heart Association and the International Small Animal Cardiac Health Council (ISACHC) -- Classes I through IV -- applied mainly to dogs already in heart failure. In 2009, the American College of Veterinary Internal Medicine (ACVIM) published a Consensus Statement in which the categories were divide into stages, beginning with MVD-affected dogs with only mitral valve murmurs (Stage B1) up to dogs inhaeart failure and for which customary medications no longer were effective (Stage D). In 2019 the ACVIM published a second Consensus Statement in which Stages B1 and B2 were completely revised and no longer make any scientific sense. In a May 2021 article, a team of Italian researchers devised a scoring system for classifying the severity of mitral valve disease (MVD or MMVD) in dogs. Its name, "MINE", is an acronym for "Mitral INsufficiency Echocardiographic score".
• New York Heart Association (NYHA) Classes of Heart Disease
This scheme was devised in 1994 for humans and has been modified to diagnose underlying cardiac disease and assessment of severity in dogs, made on the basis of the functional severity of heart failure signs, as well as physical, radiographic, and echocardiographic findings:
• Class 0:
Clinically normal dogs without a heart murmur.
• Class I:
Dogs with a heart murmur, but no radiological or echocardiographical
evidence of cardiac enlargement.
• Class II:
Dogs with echocardiographical and radiological evidence of cardiac
enlargement but no clinical or radiological evidence of pulmonary
congestion and/or pulmonary edema.
• Class III:
Dogs with nocturnal coughing and dyspnea during stress, with pulmonary
congestion and/or edema present on thoracic radiographs.
•
Class IV:
Dogs with marked respiratory distress from pulmonary congestion and
edema at rest, in addition to signs listed in Class III.
• ISACHC Classes of Heart Disease
As of 2014, the ISACHC (International Small Animal Cardiac Health Council) has classified MVD in three primary stages, with sub-categories:
• Stage Ia: Mitral/tricuspid valve murmur but no cardiac enlargement
• Stage Ib: Murmur + cardiac enlargement, but no clinical signs
• Stage II: Mild heart failure or when heart failure is controlled by therapy
• Stage III: Advanced heart failure with clinical signs, including respiratory distress or marked ascites and exercise intolerance
Prior to 2009, this classification system for heart failure most familiar to veterinarians was the published by the ISACHC:
• Class I describes patients with asymptomatic heart disease (eg, CVHD is present, but no clinical signs are evident even with exercise).
• Class II describes patients with heart disease that causes clinical signs only during strenuous exercise.
• Class III describes patients with heart disease that causes clinical signs with routine daily activities or mild exercise.
• Class IV describes patients with heart disease that causes severe clinical signs even at rest.
RETURN TO TOP
• 2009 ACVIM Consensus Statement

 In
a very important 2009 "Consensus
Statement" published by a panel of ten board certified veterinary
cardiologists (C. Atkins [left], J.
Bonagura, S. Ettinger, P. Fox, S. Gordon, J. Haggstrom, R. Hamlin, B. Keene,
V. Luis-Fuentes, and R. Stepien) of the American College of Veterinary Internal Medicine (ACVIM), they create a new classification of stages of
MVD.
In
a very important 2009 "Consensus
Statement" published by a panel of ten board certified veterinary
cardiologists (C. Atkins [left], J.
Bonagura, S. Ettinger, P. Fox, S. Gordon, J. Haggstrom, R. Hamlin, B. Keene,
V. Luis-Fuentes, and R. Stepien) of the American College of Veterinary Internal Medicine (ACVIM), they create a new classification of stages of
MVD.
They stated:
"The new system describes 4 basic stages of heart disease and failure:
"Stage A identifies patients at high risk for developing heart disease but that currently have no identifiable structural disorder of the heart (e.g., every Cavalier King Charles Spaniel without a heart murmur)." (So, Stage A means the dog does not have any MVD murmur but that its breed is at high risk for developing MVD.)
"Stage B identifies patients with structural heart disease (e.g., the typical murmur of mitral valve regurgitation is present), but that have never developed clinical signs caused by heart failure. Because of important clinical implications for prognosis and treatment, the panel further subdivided Stage B into Stage B1 and B2.
"Stage B1 refers to asymptomatic patients that have no radiographic or echocardiographic evidence of cardiac remodeling [enlargement] in response to CVHD." (So, Stage B1 means the dog has a murmur but no enlargement of the heart.)
"Stage B2 refers to asymptomatic patients that have hemodynamically significant valve regurgitation, as evidenced by radiographic or echocardiographic findings of left-sided heart enlargement." (So, Stage B2 means the dog has both a murmur and enlargement of the left side -- atrium and/or ventricle -- of the heart.)
"Stage C denotes patients with past or current clinical signs of heart failure associated with structural heart disease. Because of important treatment differences between dogs with acute heart failure requiring hospital care and those with heart failure that can be treated on an outpatient basis, these issues have been addressed separately by the panel. Some dogs diagnosed with heart failure for the first time may have severe clinical signs requiring aggressive therapy (e.g,, with additional afterload reducers or temporary ventilatory assistance) that more typically would be reserved for those with refractory disease (see Stage D)." (So, Stage C means the dog is or has been in heart failure but drugs are enabling the heart to compensate for that failure.)
"Stage D refers to patients with end-stage disease with clinical signs of heart failure caused by CVHD [MVD]that are refractory to 'standard therapy' (defined later in this document). Such patients require advanced or specialized treatment strategies in order to remain clinically comfortable with their disease. As with Stage C, the panel has distinguished between animals in Stage D that require acute, hospital-based therapy and those that can be managed as outpatients." (So, Stage D means the dog is in heart failure and drugs no longer allow the heart to compensate for that failure.)
The typical progression for MVD-affected cavaliers is to pass from Stage B1 to B2 to C to D. For many other breeds, especially if the onset of MVD is late in life, the progression may stop at Stage B2, with the dog never reaching heart failure. Rarely, a cavalier may by-pass Stage B2 -- the enlargement stage -- and proceed directly from only a murmur in Stage B1 to heart failure in Stage C. This could occur if one or more of the dog's mitral valve's major chordae tendineae should suddenly rupture before any enlargement takes place. It is even possible for a chord to rupture before a murmur is detected (skipping Stage B1), since degeneration occurs in both the leaflets and the chords of mitral valves.
RETURN TO TOP
• 2019 ACVIM Consensus Statement
In 2019, the ACVIM issued a new "Consensus Statement" in which it completely changed the definitions of Stage B1 and B2. According to the 2019 definition of Stage B1, the heart either has no enlargement OR either the left atrium (LA) OR the left ventricle (LV) is enlarged, but not both of them, OR, if both of them are enlarged, one of both of them are not enlarged up to the minimum dimensions of the 2019 version of Stage B2.
The 2019 definition of Stage B2 requires that both the left atrium and left ventricle be enlarged by specific minimum dimensions. So, the MVD-affected dog's heart can be enlarged and still be in Stage B1, even if both the LA and LV are enlarged but not large enough to meet those minimum dimensions. And, the MVD-affected dog's heart could normal sized and not enlarged at all, but if its LA and LV meet those minimum dimensions, it will be in Stage B2. This is the result of the 2019 ACVIM Consensus Statement panel assuming (without any scientific evidence to support that assumption) that all breeds of dogs have the same sized hearts when both normal sized and enlarged.
Stage B2: Any MVD-affected dog with these minimum measurements:
• murmur intensity >3/6;
• echocardiographic LA:Ao ratio in the right-sided short axis view in early diastole >1.6;
• left ventricular internal diameter in diastole, normalized for body weight (LVIDDN) >1.7;
• breed-adjusted radiographic vertebral heart size (VHS) > 10.5.
• Ideally, all these criteria should be met before initiating treatment, because treatment represents a lifelong commitment. Of these criteria, echocardiographic evidence of left atrial and ventricular enlargement meeting or exceeding these criteria is considered to be the most reliable way to identify dogs expected to benefit from treatment.
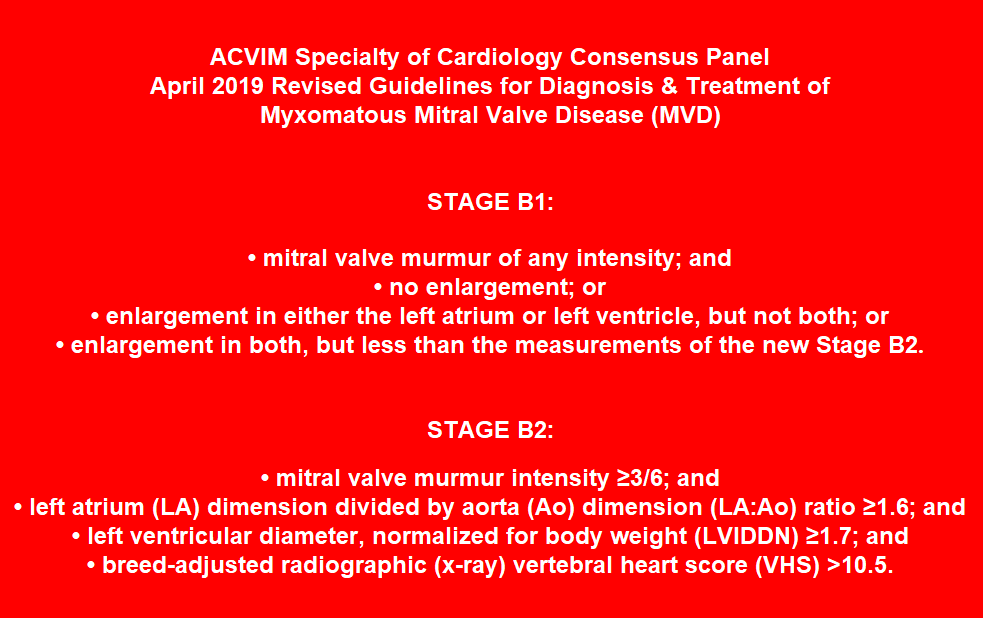
The ACVIM's definitions of Stages B1 and B2 are unscientific nonsense
The 2019 ACVIM Consensus Statement's definitions of Stages B1 and B2 are pure, unscientific nonsense, for these reasons:
 Thus far, no species-wide studies of the parameters for determining
if a dog's heart is enlarged have been published which justify applying
a single measurement as being the dividing line between a normal-sized
left atrium or left ventricle and an enlarged left atrium or left
ventricle, and dozens of
breed-specific articles contradict it. Nevertheless, the ACVIM
Consensus Statement authors apply two species-wide dividing
lines and conclude that if any dog's heart exceeds them, the dog
necessarily has an enlarged heart, and if either of the dog's left chambers does
not exceed them, then that dog's heart necessarily is of normal size. They make a mockery of the so-called
"evidence-based" practice of veterinary medicine.
Thus far, no species-wide studies of the parameters for determining
if a dog's heart is enlarged have been published which justify applying
a single measurement as being the dividing line between a normal-sized
left atrium or left ventricle and an enlarged left atrium or left
ventricle, and dozens of
breed-specific articles contradict it. Nevertheless, the ACVIM
Consensus Statement authors apply two species-wide dividing
lines and conclude that if any dog's heart exceeds them, the dog
necessarily has an enlarged heart, and if either of the dog's left chambers does
not exceed them, then that dog's heart necessarily is of normal size. They make a mockery of the so-called
"evidence-based" practice of veterinary medicine.
The consequence to the MVD-affected dogs of these revisions to the defintions of Stage B1 and B2 is that the dogs with enlarged hearts in Stage B1 are not to be medicated for their MVD, and dogs with normal-sized hearts in Stage B2 are eligible to be medicated. "Precision care" or "personalized medicine" is defined as "providing the right therapy for the right patient at the right time." Obviously, the ACVIM does not believe in this pinpoint approach to veterinary care when it comes to treating dogs diagnosed with mitral valve disease.
This new definition of Stage B2 is the same as that used in the 2016 EPIC Study (which was fully financed by Boehringer Ingelheim, the exclusive patent holder for Vetmedin/pimobendan), with the exception that the VHS > 10.5 was not "breed-adjusted" in the EPIC Study. The 2019 consensus panel unanimously recommends that any Stage B2 dog be treated with pimobendan at a dosage of 0.25-0.3 mg/kg twice a day. No other medication is recommended for Stage B2 by more than half the panel. In the article's Conflict of Interest Declaration, all ten of the panelists admit that they have been consultants for Vetmedin's manufacturer, Boehringer Ingelheim, and presumably have been financially rewarded for their services to that company.
In a June 2022 article, Drs. Mark Rishniw and Donald J. Brown studied the echocardiograms of 1,124 dogs with healthy, normal-sized hearts, including 59 cavaliers. Their goal was to determine if the EPIC Study's and 2019 ACVIM Consensus Statement's species-wide definition of enlarged left ventricles was accurate, and if not, what other criteria should be applied. They concluded that the EPIC and ACVIM definition (LVIDDN > 1.7) was "arbitrarily chosen" and that over 10% of healthy dogs in the study, including the cavaliers, would be mis-classified as having enlarged left ventricles (LV) when in fact they had normal-sized LVs. They single out five specific breeds, including the CKCS, as having larger LVs than average dogs of similar size. They state:
"We should note, however, that the definition of LV enlargement proposed by both the EPIC trial and, subsequently, by the ACVIM Consensus panel, is not a reference value, but a somewhat arbitrarily chosen threshold for inclusion in the EPIC trial. Therefore, clinicians adhering to these guidelines should be cautious in ascribing LVIDDN that exceed the threshold value of 1.7 as being indicative of LV enlargement. Furthermore, clinicians should note that the ACVIM Stage B2 classification requires enlargement of both the left atrium (to a LA:Ao>1.6) and enlargement of the left ventricle. How many dogs with mild left atrial enlargement, but without LV enlargement, might consequently be misclassified as having cardiomegaly remains unknown."
The investigators recommend that such breeds as the CKCS "likely require breed-specific reference intervals for LVIDDN to correctly identify left ventricular enlargement."
In a March 2024 article, French researchers studied the echocardiographic measurements of 155 cavaliers (128 diagnosed with mitral valve disease and 27 without MVD), comparing linear measurements of the left atrium (LA) with volumetric measurements, including monoplane and biplane Simpson's methods of discs (SMOD) and area-length methods (ALM). They made numerous observations, the most significant of which being:
• The 2019 ACVIM Consensus Statement's (and 2016 EPIC Study's) classification cut-offs of LA enlargement "do not correctly fit with the CKCS breed regarding both LA and LV sizes. Therefore, in the present study, CKCS breed-specific reference intervals (instead of the ACVIM multi-breed cut-offs) were used to define LA and LV enlargement".
• In healthy CKCS dogs (ACVIM stage A), the median LA:Ao ratios were ... 1.02 (0.90-1.12) at end-systole.
• 37% of the 2019 ACVIM stage B1 CKCSs demonstrated LA enlargement.
• "Regarding LA and LV dilation criteria, there is now scientific evidence that the proposed multibreed echocardiographic ACVIM cut-offs do not perfectly fit for several specific breeds, and the use of breed-specific reference intervals would be more appropriate. As recently demonstrated by Rishniw and Brown, approximately 10% of healthy CKCSs would be misclassified as having LV enlargement using the ACVIM-recommended scaling exponent (0.294) and the cut-off of 1.7 for normalized end-diastolic LV internal diameter. The study published by Misbach et al dedicated to the establishment of echocardiographic reference intervals in a large population of healthy CKCSs also demonstrated the discrepancy between their LV breed-specific reference intervals (assessed according to the Clinical Laboratory and Standard Institute recommendations) and the predictive reference intervals obtained in the same population using Cornell's formula."
• "Regarding LA size, CKCS dogs seem to have smaller end-diastolic and end-systolic LA dimensions than most other breeds."
• "Based on these data, the end-systolic LA:Ao ratio cut-off value of 1.6 proposed by the 2019 consensus ACVIM statement to detect end-systolic LA enlargement in all-breed dogs appears inappropriate (i.e., too high) for CKCSs, with a risk of missing some mild LA dilations."
• "[A] sub-population [31 -- 37%] of ACVIM B1 dogs with normal LA/Ao ratios actually showed LA dilation detected by LA volume calculation, which was not apparent when applying end-diastolic and end-systolic LA:Ao ratios."
• "Additionally, among the 31 CKCSs with normal end-systolic LA:Ao ratio but increased LASvol/BW volume (Fig 3), 19/31 (61%) showed increased end-diastolic LV diameter. In other words, these 19 dogs were characterized by mitral valve regurgitation severe enough to have induced both LA and LV dilation (with a median RF value of 38%). Thus, according to the 2019 ACVIM classification, these dogs may actually belong to ACVIM stage B2."
• "LA volumetric quantification should the method of first choice for assessing LA size particularly to stratify ACVIM stage B dogs. Additionally, the early identification of LA enlargement in stage B dogs is of practical interest, as these dogs at risk for decompensation may benefit from a closer longitudinal follow-up than others."
Similarly, In a May 2024 article by Italian cardiology investigators, they reported on their study of 198 cavaliers and their hearts' atrial dimensions and functions at the different stages of MVD as defined by the ACVIM's 2019 MVD Consensus Statement -- Stages A, B1, B2, C, and D. They obtained several echocardiograph measurements in addition to the left-artium-to-aorta (LA/Ao) ratio prescribed by the ACVIM's Consensus Statement. These measurements included left atrial anteroposterior diameter normalized for body weight (LADn) and left atrial P volume (LAVp). When comparing cavaliers in Stage A and Stage B1, they report finding significant atrial enlargement in Stage B1 dogs. Specifically, they found that Stage B1 dogs have increased LADn, suggesting that enlargement of the left atrium (LA) already has begun in Stage B1 cavaliers, even though the ACVIM's 2019 Consensus Statement insists that Stage B1 cavaliers have normal sized LAs when in fact their LAs already have been enlarging. They also find that a notable difference in the functioning of the LA between Stages A and B1, and that the active and passive left atrial emptying fractions are useful in detecting dysfunction of the LA in MVD-affected CKCSs. See also this September 2024 version of their study.
Numerous veterinary journal articles have concluded that there are significant breed-specific variations in echocardiographic indices measuring left chamber dimensions, when determining whether or not an MMVD-affected dog's left atrium or left ventricle is, in fact, enlarged. Here are 34 examples (with emphasis added):
(1) Morrison, S.A., et al., Effect of Breed and Body Weight on Echocardiographic Values in Four Breeds of Dogs of Differing Somatotype. J. Vet. Intern. Med. 1992;6:220-224:
"This study demonstrated that breed, in addition to weight, is an important factor in the determination of normal echocardiographic measurements for dogs."
(2) Snyder, P.S., et al., A Comparison of Echocardiographic Indices of the Nonracing, Healthy Greyhound to Reference Values from Other Breeds. Vet. Rad. & Ultra. 1995;36(5):387-392:
"Adult nonracing greyhounds were shown to have increased left ventricular cavity dimensions, systolic time intervals and wall thicknesses as compared to mongrels and various other dog breeds. ... Our finding that greyhounds have cardiac dimensions that are apparently larger than other dogs of similar weight is supported by earlier gross anatomic studies of greyhounds."
"In summary, normal nonexercised adult greyhounds have increased left ventricular cavity dimensions, systolic time intervals and left ventricular and septal wall thicknesses that differ markedly from reported normal echocardiographic values derived from mixed breed and other dogs. ... Despite correction of the data for body weight or surface area, the differences remained, suggesting that breed and body conformation may need to be considered in addition to heart rate when interpreting echocardiographic studies in the dog. Nonetheless, caution should be employed in utilizing generic reference ECHO values in the evaluation of echocardiographic studies in greyhounds."
(3) P.K. Della Torre, et al., Echocardiographic measurements in Greyhounds, Whippets and Italian Greyhounds -- dogs with a similar conformation but different size. Austr. Vet. J. 2000;78(1):49-55:
"The results from this study, taken together with previous reports, suggest Greyhounds, Whippets and Italian other breeds of comparable body size. This possibly reflects selection for athletic performance. This study adds further weight to the notion that breed variation precludes the determination of an effective reference range for echocardiographic measurements widely applicable to all purebred and crossbred dogs."
(4) O'Leary, C.A., et al., Echocardiographic parameters in 14 healthy English Bull Terriers. Austr. Vet. J 2003;81(9):535-542:
"In this study, the prediction interval for LA:Ao calculated from 2D long axis measurements in English Bull Terriers was 1.3 to 2.1."
"The dogs in this study had increased LA diameter, LV wall thickness and AoV, and decreased aortic root diameter compared with other breeds of similar body size. In addition, FS% was lower than in dogs of comparable size, and more similar to the values reported from giant breeds."
(5) Muzzi, R.A.L., et al., Echocardiographic indices in normal German shepherd dogs. J. Vet. Sci. 2006;7(2):193-198:
"Adult German shepherd dogs were shown to have larger left ventricular dimensions and wall thicknesses than other small breeds of dogs. These differences suggest that the breed and body conformation in addition to the heart rate may need to be considered when interpreting echocardiographic studies in dogs. Caution should be taken in extending these generic reference echocardiographic values to an evaluation of echo studies."
(6) Kayar, A., et al., M-Mode Echocardiographic Parameters and Indices in The Normal German Shepherd Dog. Vet. Rad. & Ultra. 2006;47(5):482-486:
"Although some normal echocardiographic values for dogs have been published, there is variation based on breeds, body size, and somatotype. Indeed, echocardiographic reference ranges derived from some dog breeds may be misleading for others."
(7) Bavegems, V., et al. Echocardiographic Reference Values in Whippets. Vet. Rad. & Ultra. 2007;48(3):230-238:
"We confirm that whippets have a larger LVD, a thicker IVS and LVW, and a lower FS than expected for dogs of comparable body weight. The clinician should be aware of these specific differences in whippets to avoid misdiagnosis of cardiac dilation, hypertrophy, and/or myocardial failure in these dogs."
(8) Voros, K., et al., M-Mode and Two-Dimensional Echocardiographic Reference Values for Three Hungarian Dog Breeds: Hungarian Vizsla, Mudi and Hungarian Greyhound. Acta Vet. Hungarica. 2009;57(2):217-227:
"Breed has been demonstrated to be an important factor, influencing intracardiac parameters in addition to body weight in dogs. Therefore, the need for normal breed-specific reference ranges has been emphasised for comparison of pathological changes caused by cardiac disorders."
(9) Jacobson, J.H., et al., An echocardiographic study of healthy Border Collies with normal reference ranges for the breed. J. Vet. Cardiol. 2013;15(1):123-130:
"There are differences in some echocardiographic parameters between healthy Border Collies and the general dog population, and the echocardiographic reference ranges provided in this study should be used as breed specific reference values for Border Collies."
"Although echocardiographic values for the general dog population have been published, it is now known that there can be variation based on breed, body size, somatotype and body surface area. Echocardiographic reference ranges derived from some dog breeds may be misleading for others, limiting their clinical usefulness."
"There are over 37 papers investigating breed specific and generic echocardiographic parameters. These studies add support to the belief that breed variation precludes the determination of a single reference range for echocardiographic measurements that may be widely applied to all purebred and crossbred dogs."
(10) M. Hollmer, et al. Left atrial volume and phasic function in clinically healthy dogs of 12 different breeds. Vet. J. September 2013; doi: 10.1016/j.tvjl.2013.05.045:
"We provided breed-specific reference ranges for 12 breeds and clinical reference ranges for use in all dogs based on BW. ... These values of LA volume might provide a better basis for assessing LA size than linear LA dimensions when examining dogs with heart disease, since small increases in linear dimensions can reflect significant increases in LA volume due to the asymmetrical nature of LA dilatation. Since body size is a major determinant of LA size, LA volume should be indexed to a measure of body size to allow meaningful comparison."
"Even when the effects of body size were taken into account, these data showed significant breed differences for LA volume. Thus, LA volume measurements in dogs of the same weight can differ slightly among breeds. It would be ideal to establish breed-specific reference values due to the differences in type of breed, size and conformation of the thorax."
(11) Gugjoo, M.B., et al., Reference values of M-mode echocardiographic parameters and indices in conscious Labrador Retriever dogs. Iranian J. Vet. Res. 2014;15(4):341-346:
"The echocardiographic indices show significant breed variations and it is important to know the normal echocardiographic values for each dog breed considering the influence of body weight on the established echocardiographic values."
(12) Misbach, C., et al. Echocardiography and conventional Doppler examination in clinically healthy adult Cavalier King Charles Spaniels: Effect of body weight, age, and gender, and establishment of reference intervals. J. Vet. Cardiol. 2014;16(2):91-100:
"Due to the large variations in size and conformation within the canine population, significant breed and body weight (BW) effects on echocardiographic variables have been demonstrated, mainly for left ventricular (LV) M-mode measurements. Body weight independent differences among breeds have also been reported. For these reasons, the breed must be considered when establishing echocardiographic RI [reference intervals] in the dog."
"In conclusion, although predictive RI assessed according to Cornell's formula seem to be acceptable for CKCS dogs, the present results suggest that establishing specific RI for echocardiographic and Doppler variables in this breed is relevant."
(13) Limm, C.K., et al., Two-dimensional left atrium-to-aorta ratios and left ventricular M-mode transthoracic echocardiographic measurements in clinically normal adult Dachshunds. AJVR 2016;77(4):374-382:
"The mean LA:Ao for the clinically normal Dachshunds of the present study was markedly different from the mean LA:Ao for clinically normal CKCSs in another study, even though the body weights for the Dachshunds (range, 5.0 to 12.6 kg) and CKCSs (5.5 to 11.9 kg) were similar. The LA:Ao as determined by the diameter method was < 1.67 for all the Dachshunds of the present study and < 1.28 for all the CKCSs of that other study."
(14) Duler, L., Interreader agreement of radiographic left atrial enlargement in dogs and comparison to echocardiographic left atrial assessment. J. Vet. Cardiol. August 2018; doi: 10.1016/j.jvc.2018.07.004:
"Our results also suggest that there are limitations to using a single linear dimension (LA:Ao) to assess LA size. The LA is a complex cardiac structure that can enlarge in multiple planes, so single measurement may not accurately reflect actual LAE. This is supported by the stronger agreement of radiographic LAE with LAV, rather than LA:Ao. Owing to the eccentric manner in which atrial enlargement occurs, large differences in overall LA size may be misrepresented by a small range of LA:Ao values. Enlargement of the LA primarily in the dorsal-ventral orientation may not be apparent in the single plane LA:Ao measurement, while enlargement in multiple directions is incorporated into the LAV value by tracing the LA border. In addition, dividing by the aortic dimension is an attempt to index LA size to a cardiac structure that theoretically does not change much in disease states. However, the size of the aorta may vary throughout the cardiac cycle [15]. While LA:Ao is less time consuming than volumetric measurements, this study and other recent veterinary studies emphasize the limitations of the LA:Ao measurement."
(15) Garncarz, M, et al., Reference intervals for transthoracic echocardiographic measurements in adult Dachshunds. Polish J. Vet. Sci. 2018;21(4):779-788:
"In recent years many breed specific echocardiographic parameters have been published for canines showing significant differences between these measurements in different dog breeds. This underlines the necessity to prepare reference values for specific breeds."
"It appears that the different size heart in the Dachshunds could simply be a characteristic of the breed, possibly resulting from a smaller body size compared to other dog breeds, a different chest conformation, or even the influence of systemic blood pressure. ... To minimize the chance of erroneously accepting an enlarged left ventricle as normal, Dachshund specific values should be used. This is especially important when classifying disease stage in Dachshunds with chronic mitral valve disease according to the most recent guidelines (Atkins et al. 2009) as this disease readily leads to chamber enlargement, including the left atrium and left ventricle."
(16) Visser, L.C., et al, Echocardiographic quantitation of left heart size and function in 122 healthy dogs: A prospective study proposing reference intervals and assessing repeatability. J. Vet. Intern. Med. 2019; doi: 10.1111/jvim.15562:
"Unlike in human medicine, recommended standards for quantitation of cardiac chamber size and function by echocardiography in dogs do not currently exist."
"Currently, no accepted standard exists on how to determine RIs [reference intervals] (ie, clinical cutoffs to help distinguish normal from abnormal) for echocardiography data sets in healthy dogs."
"Thus, common approaches for indexing linear measurement to body size have included breed-specific reference values ...".
(17) Rishniw, M., Two-dimensional echocardiographic left-atrial-to-aortic ratio in healthy adult dogs: a re-examination of reference intervals. J. Vet. Cardiol. November 2019;doi: 10.1016/j.jvc.2019.11.001:
"Twenty-eight dogs [12%] had LA:AoMAX (RPSA) >1.6, and seven dogs [3%] had LA:AoMAX (RPSA) >1.7. ... In our current study, approximately 10% of apparently healthy dogs exceeded this [LA:Ao = 1.6] limit. ... Our finding that LA:AoMAX, obtained from the right parasternal short-axis view, might exceed 1.6 in approximately 10% of dogs has clinical implications. ... We have shown that previously published upper reference limits for LA:AoMAX might be slightly low and that LA:Ao obtained at different time-points or from different views are not interchangeable."
(18) Stack, J.P., et al., Reference Intervals and Echocardiographic Findings in Leonberger Dogs. J. Vet. Cardiol. 2020; doi: 10.1016/j.jvc.2020.03.006:
"Currently, the normal echocardiographic variables of Leonbergers are unknown. Although general canine reference ranges have been published, the large number of studies evaluating breed-specific data supports the notion that the general reference ranges have limitations due to the marked phenotypic variations across canines."
(19) Gerhard Wess, et al., Echocardiographic reference intervals for volumetric measurements of the left ventricle using the Simpson's method of discs in 1331 dogs. J. Vet. Intern. Med. March 2021; doi: 10.1111/jvim.16089:
"Veterinary cardiologists face the problem of dealing with many different breeds and therefore a wide range of different body and BWs [body weights]. ... The optimal situation would be to have breed-specific RIs, generated from a large healthy population."
(20) Dutton, E., et al., Echocardiographic reference intervals in healthy UK Deerhounds and prevalence of preclinical dilated cardiomyopathy: a prospective, longitudinal study. J. Vet. Cardiol. April 2021; doi: 10.1016/j.jvc.2021.04.001:
"Healthy Deerhounds have higher LVDd, LVDs and EDVI compared with other breeds. This study provides ECHO RIs [reference intervals] for Deerhounds; sex or BW [body weight] RIs should be used when screening."
(21) Tommaso Vezzosi, et al. Reference intervals for transthoracic echocardiography in the American Staffordshire Terrier. J. Vet. Med. Sci. April 2021; doi: 10.1292/jvms.20-0622:
"Regarding echocardiographic variables, the mean LVIDDn in our AST population was significantly higher than values reported in a range of canine breeds. ... Regarding the LA/Ao, the upper reference limit in our AST population was 1.75, with 6/57 (10%) dogs with a LA/Ao >1.6. ... The LA/Ao is the most widely used echocardiographic index to evaluate left atrial dimension in dogs, and several studies have used the upper reference limit of 1.6 to define left atrial enlargement. However, our results are in line with a study demonstrating that in around 10% of dogs, in particular in Boxers and Beagles, a small aortic root is present, which leads to an increase in the LA/Ao up to an upper reference limit of 1.73."
"These breed-specific echocardiographic features should be taken into consideration for an accurate echocardiographic interpretation and screening in the AST."
(22) Patata, V., et al., Echocardiographic parameters in 50 healthy English Bulldog: Preliminary Reference Intervals. J. Vet. Cardiol. May 2021; doi: 10.1016/j.jvc.2021.04.010:
"In our sample of EB [English bulldogs], the LA:Ao ratio in the short axis plane exceeded the previous upper reference limit of <1.6 in a significant number of dogs (12%). ... Thus, the use of breed-specific echocardiographic reference intervals is ideal for appropriate structural and functional assessment. ... Additionally, breed-specific reference intervals offer the possibility to develop better echocardiographic screening programs in the EB. ... Further studies comparing indexed cardiac dimensions and geometry among canine breeds with different thoracic somatotype may be useful."
(23) Oktawia Szpinda, et al. Impact of selected individual dog traits on echocardiographic parameters obtained in 1-dimensional (M-mode) and 2-dimensional (2D) imaging. Canadian J. Vet. Res. April 2021;85(2):112-118:
"It is important to establish echocardiographic values for individual dog breeds, not only for primary measurements such as left ventricular dimension or left atrial dimension, but also for secondary measurements. ...
"Due to the wide variety of dog breeds and inhomogeneous research groups, echocardiographic measurements should be established individually for each breed based on a population study. This will allow for a more accurate interpretation of the results obtained in the echocardiographic examination and lead to earlier diagnosis of heart disease. ...
"The great variety of dog breeds means that echocardiographic findings should be individually interpreted rather than establishing reference ranges for each breed in population studies. This will allow for a more accurate interpretation of the results obtained in the echocardiographic examination and consequently lead to earlier diagnosis of changes in myocardial morphology."
(24) Murat Vurucu, et al. An echocardiographic study of breed-specific reference ranges in healthy French Bulldogs. Vet. Radiol. & Ultrasound. May 2021; doi: 10.1111/vru.12997:
"Reference values obtained from breed-specific echocardiographic studies have been reported to have a significant difference compared to the general population of healthy different dog breeds. Therefore, breed-specific echocardiographic reference ranges may be more helpful in avoiding the misinterpretation of echocardiographic findings. During an evaluation without considering the breed-specific echocardiographic reference values, a normal heart structure and/or measurement could end up being falsely interpreted as either large or small or increased or decreased when compared to the general non-breed-specific reference ranges. These misinterpretations could lead to a misdiagnosis or an underdiagnosis of an underlying cardiac problem resulting in unnecessary/wrong treatment and/or no treatment."
"An echocardiographic evaluation of French Bulldogs based on general or different dog breed population reference ranges may result in misdiagnosis (such as an early phase of dilated cardiomyopathy or heart failure) because this breed has a different chest structure, cardiac silhouette, and left ventricular internal dimensions compared to other breeds. ... Normal heart sizes and shapes show substantial variation based on dog breed. Breed-specific reference ranges should be taken into consideration when evaluating the heart."
(25) Liva Vatne, et al. The effects of activity, body weight, sex and age on echocardiographic values in English setter dogs. J. Vet. Cardiol. August 2021; doi: 10.1016/j.jvc.2021.08.003:
"Our study provides transthoracic echocardiographic values for English setter dogs, showing that they have larger hearts than many other breeds and fall outside of generic reference intervals for dogs. ... Breed specific reference intervals improve echocardiographic interpretation and thereby reduce misdiagnoses. ... The study provides breed specific transthoracic echocardiographic values for English setter dogs, thereby contributing to improve diagnostic assessment of cardiac health in this breed."
(26) Mark Rishniw, Donald J. Brown. The ACVIM consensus statement definition of LV enlargement in myxomatous mitral valve disease does not always represent LV enlargement. J. Vet. Cardiol. June 2022; doi: 10.1016/j.jvc.2022.06.004:
"The EPIC study employed an essentially arbitrary value of LVIDDN to dogs subsequently randomized to treatment with pimobendan or placebo and showed that dogs with LVIDDN>1.7 (and LA:Ao>1.6 and a VHS>10.5V) generally do better with the drug than without. ... The criterion for left ventricular enlargement used by the EPIC investigators for study inclusion has never been critically evaluated as indicative of left ventricular enlargement. ...
"We should note, however, that the definition of LV enlargement proposed by both the EPIC trial and, subsequently, by the ACVIM Consensus panel, is not a reference value, but a somewhat arbitrarily chosen threshold for inclusion in the EPIC trial. Therefore, clinicians adhering to these guidelines should be cautious in ascribing LVIDDN that exceed the threshold value of 1.7 as being indicative of LV enlargement. Furthermore, clinicians should note that the ACVIM Stage B2 classification requires enlargement of both the left atrium (to a LA:Ao>1.6) and enlargement of the left ventricle. How many dogs with mild left atrial enlargement, but without LV enlargement, might consequently be misclassified as having cardiomegaly remains unknown.
"For example, if a dog has a LVIDDN that consistently oscillates around 1.3 (within 0.1 units), an increase to 1.6 could indicate left ventricular enlargement for that dog, despite being within the population-based reference interval."
(27) Noriko Isayama, et al. Reference Values of M-mode Echocardiographic Parameter in Adult Toy Breed Dogs. Front. Vet. Sci. June 2022; doi: 10.3389/fvets.2022.918457:
"The upper limit of the prediction interval for breeds weighing <5 kg was much lower than the value currently applied. ... Taking all 86 dogs into account, when we calculated LVIDDN as measured LVIDD/BW0.294, according to the ACVIM consensus statement, the median LVIDDN value was 1.3215 and the mean value was 1.3204, the observed minimum and maximum were 0.9214 and 1.6072, respectively, while in the first and third quartiles, the values were 1.2472 and 1.4140, respectively. Notably, none of these values approached 1.7, the threshold value proposed previously, and was clinically followed by the ACVIM consensus statement. A natural corollary of this observation is that since the normal range for dogs <5 kg is <1.7, the possibility is high that by the time these toy breed dogs start receiving treatment, according to the current guidelines of diagnosis criteria of stage B2 of MMVD, they already reach a very advanced stage of the disease. Previous reports, including the EPIC study, focused mainly on dogs with BW >4 kg, and the diagnostic criteria for toy breed dogs remain mostly unexplored. Therefore, along with diagnostic criteria, we should consider starting treatment as B2 for dogs weighing <5 kg with an LVIDDN of >1.6."
(28) M. Brloznik, et al. Echocardiographic parameters in French Bulldogs, Pugs and Boston Terriers with brachycephalic obstructive airways syndrome. BMC Vet. Res. February 2023; doi: 10.1186/s12917-023-03600-9:
"The results of this study showed significant differences in echocardiographic parameters between the dogs of the three brachycephalic breeds and non-brachycephalic dogs. In addition, there were significant differences in echocardiographic parameters between dogs with and without signs of BOAS. ... We found significant differences in echocardiographic parameters between dogs of the three brachycephalic breeds and non-brachycephalic dogs, implying that breed-specific echocardiographic reference values should be used in clinical practice. In addition, significant differences were observed between brachycephalic dogs with and without signs of BOAS. The observed echocardiographic differences suggest higher right heart diastolic pressures affecting right heart function in brachycephalic dogs with and without signs of BOAS, and several of the differences are consistent with findings in OSA patients. Most of the changes of the heart morphology and function can be attributed to brachycephaly alone and not to the symptomatic stage."
(29) Oktawia Szpinda, et al., Cardiological Reference Intervals in Adult American Staffordshire Terrier Dogs. Animals. July 2023; doi: 10.3390/ani13152436:
"The echocardiographic examination performed on the AST dogs showed that this breed is characterized by a symmetrical enlargement of the cardiac silhouette compared to generally accepted RIs. AST dogs had larger LVDd, LVDs, and LA, which were similar to those measured in larger dog breeds of 30-40 kg, such as the Doberman Pincher, with a ranging BW of 26-53 kg, as well as Boxers, and German Shepherds. ... AST dogs also had smaller Ao diameters; similar sizes of the Ao were examined in Labrador Retrievers and Golden Retrievers. ...
"Due to the great variety of dog breeds subjected to echocardiography examination, RIs for echocardiographic examination for individual dog breeds have been increasingly published in the literature. The authors of these publications have indicated that creating standards for individual breeds based on specific studies of entire populations of dogs of one breed is much more accurate, because it takes into account their breed characteristics--body structure, conformation, and natural physical activity, not only BW. ...
"Our study indicates that dogs of the AST breed should be individually approached and the condition of their heart should be assessed against breed-specific RI. Such action will increase the chance to detect even minor deviations from the breed-specific RI and prevent misdiagnosis."
(30) Maria Cerbu. et al. M-Mode Echocardiography in Canine Veterinary Practice: A Comprehensive Review of Left Ventricular Measurements in 44 Different Dog Breeds. Animals. September 2023; doi: 10.3390/ani13182986:
"To ensure accurate interpretation of echocardiographic examinations in dogs, it is crucial to consider breed-specific reference values. Failing to do so can lead to misinterpretations, falsely indicating heart enlargement or altered activity when compared with general reference ranges, potentially resulting in misdiagnosis and inappropriate treatment."
(31) D. Dickson, et al. Differences in Left Ventricular Enlargement Secondary to Chronic Volume Loading Between English Springer Spaniels and Two Similar Sporting Breeds. August 2023; doi: 10.1016/j.jvc.2023.06.001:
"We found that English springer spaniels develop more pronounced left ventricular eccentric hypertrophy when exposed to chronic mitral regurgitation compared with two similarly sized athletic breeds. ... English springer spaniels have greater left ventricular dimensions when exposed to chronic mitral regurgitation, compared with Border collies and Labrador retrievers. ... English springer spaniels differ in their left ventricular morphology from two other sporting breeds, supporting previous studies that they have a unique cardiac morphotype."
(32) Valerie Chetboul, et al. Volumetric quantification identifies some left atrial dilations undetected by left atrium:aorta ratio measurements: A prospective echocardiographic study in 155 Cavalier King Charles Spaniels with and without degenerative mitral valve disease. PLoS One. March 2024; doi: 10.1371/journal.pone.0300827. eCollection 2024:
"[T]hese multibreed echocardiographic ACVIM cut-offs do not correctly fit with the CKCS breed regarding both LA and LV sizes. Therefore, in the present study, CKCS breed-specific reference intervals (instead of the ACVIM multi-breed cut-offs) were used to define LA and LV enlargement, as explained above. ... Regarding LA and LV dilation criteria, there is now scientific evidence that the proposed multibreed echocardiographic ACVIM cut-offs do not perfectly fit for several specific breeds, and the use of breed-specific reference intervals would be more appropriate. As recently demonstrated by Rishniw and Brown, approximately 10% of healthy CKCSs would be misclassified as having LV enlargement using the ACVIM-recommended scaling exponent (0.294) and the cut-off of 1.7 for normalized end-diastolic LV internal diameter. The study published by Misbach et al dedicated to the establishment of echocardiographic reference intervals in a large population of healthy CKCSs also demonstrated the discrepancy between their LV breed-specific reference intervals (assessed according to the Clinical Laboratory and Standard Institute recommendations) and the predictive reference intervals obtained in the same population using Cornell's formula. ... Regarding LA size, CKCS dogs seem to have smaller end-diastolic and end-systolic LA dimensions than most other breeds. ... Based on these data, the end-systolic LA:Ao ratio cut-off value of 1.6 proposed by the 2019 consensus ACVIM statement to detect end-systolic LA enlargement in all-breed dogs appears inappropriate (i.e., too high) for CKCSs, with a risk of missing some mild LA dilations. ... [A] sub-population [31 -- 37%] of ACVIM B1 dogs with normal LA/Ao ratios actually showed LA dilation detected by LA volume calculation, which was not apparent when applying end-diastolic and end-systolic LA:Ao ratios. ... Additionally, among the 31 CKCSs with normal end-systolic LA:Ao ratio but increased LASvol/BW volume (Fig 3), 19/31 (61%) showed increased end-diastolic LV diameter. In other words, these 19 dogs were characterized by mitral valve regurgitation severe enough to have induced both LA and LV dilation (with a median RF value of 38%). Thus, according to the 2019 ACVIM classification, these dogs may actually belong to ACVIM stage B2. ... LA volumetric quantification should the method of first choice for assessing LA size particularly to stratify ACVIM stage B dogs. Additionally, the early identification of LA enlargement in stage B dogs is of practical interest, as these dogs at risk for decompensation may benefit from a closer longitudinal follow-up than others."
(33) Paolo Savarino, et al. Left atrial volume and function in Cavalier King Charles spaniels at different ACVIM stages. Res. Vet. Sci. September 2024; doi: 10.1016/j.rvsc.2024.105428:
"Dogs in stage A had a smaller LA/Ao ratio than dogs in stage B2 or stage C/D, but not smaller than dogs in stage B1. ... Our study revealed a notable difference in the left atrial function between the ACVIM A and B1 stages. ... In conclusion, this study demonstrates that CKCS dogs with MMVD in class ACVIM B1 have increased LADn and left atrial volumes, suggesting the presence of atrial remodelling at a very early stage of the disease."
(34) Mara Bagardi, et al. Transthoracic echocardiographic reference intervals in 214 adult Golden retrievers. J. Vet. Med. Sci. February 2025; doi: 10.1292/jvms.24-0268:
"Golden retrievers in our study had larger diastolic left ventricular internal diameters (absolute and normalized to body weight) than those reported by Esser for the population of all non-sighthound dogs. Similarly, dogs in our study had higher systolic diameters normalized to body weight than those reported by Esser and Cornell for both the general canine population and all non-sighthound dogs. These differences are likely due to breed-specific cardiac morphology. This confirms the recent literature reporting that the LVIDDn is breed-dependent. The observed difference for LVIDd is in line with Esser: Golden retriever was found to be a "deviant breed" (more than 10% of the measurements of dogs of this breed were above or below the corresponding prediction interval) for this specific parameter. The existence of specific reference limits for breeds that do not conform to allometric models of the general population should reduce the misclassification of healthy dogs as having left ventricular enlargement. ... These breed-specific echocardiographic features should be taken into consideration for an accurate echocardiographic interpretation and screening every time the cardiologist must evaluate a Golden retriever."
As long as Stage B2 is to be the threshold of when to start pimobendan medication, then Stage B1 should be defined as a dog with a mitral valve murmur, with or without mild enlargement of either the left atrium or left ventricle or both of them. Stage B2 should be defined as a dog with a murmur and at least moderate enlargement of either of those chambers.
Our Blog: "Why do so many ACVIM cardiologists insist upon being Stuck On Stupid?"
RETURN TO TOP
• Mitral INsufficiency Echocardiographic score (MINE)
In a
May 2021 article,
a team of Italian researchers devised a scoring system
for classifying the severity of mitral valve disease (MVD or MMVD) in
dogs. Its
name, "MINE", is an acronym for "Mitral INsufficiency Echocardiographic
score". MINE score consists of four echocardiographic measurements --
(1) the left atrium-to-aorta ratio (LA/Ao); (2) the left ventricular
end-diastolic diameter normalized for body weight (LVIDDn); (3) the left
ventricular fractional shortening (FS); and (4) the E-wave transmitral
peak velocity (E-vel) -- that have been associated with survival in
MVD-affected dogs. The authors "arbitrarily selected" minimum and
maximum species-wide cutoffs for each of those
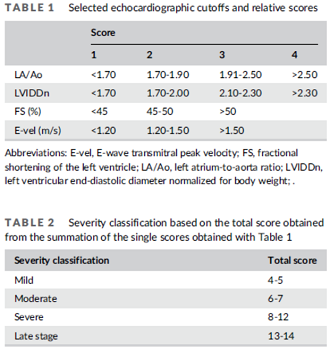 four echo measurements by the severity
of the class -- scores 1, 2, 3, and 4 -- based upon their own clinical
experiences. (See Table 1.) They then totaled each dog's scores for the
four echo measurements to arrive at a "severity classification" of:
four echo measurements by the severity
of the class -- scores 1, 2, 3, and 4 -- based upon their own clinical
experiences. (See Table 1.) They then totaled each dog's scores for the
four echo measurements to arrive at a "severity classification" of:
• Mild (total score 4-5)
• Moderate (total score 6-7)
• Severe (total score 8-12),
• Late Stage (total score 13-14).
(See Table 2.) Based upon the dogs in the study, they calculated median survival times for each of their proposed severity classes, as follows:
• Mild: 2344 days (6.42 years)
• Moderate: 1882 days (5.16 years)
• Severe: 623 days (1.71 years)
• Late Stage: 157 days (o.43 years)
They found that a MINE score above 8 (the lowest total score for the "severe" classification) was predictive of MVD being the cause of the dog's death. They concluded:
"In conclusion, we have proposed the MINE score, which is an easy-to-use echocardiographic classification of severity of MMVD, proven to be clinically effective since it is associated with survival. This classification provides prognostic information and could be useful for an objective echocardiographic assessment of MMVD. The MINE score could also be useful in identifying asymptomatic dogs with higher risk of cardiac death."
In an
August 2025 article, the
same team of Italian veterinary cardiology researchers
have re-examined their MINE formulation using 749 dogs, including 129
(17.2%) cavalier King Charles spaniels. The original MINE formula
includes our echocardiographic measurements -- (1) the left
atrium-to-aorta ratio (LA/Ao); (2) the left ventricular end-diastolic
diameter normalized for body weight (LVIDDn); (3) the left ventricular
fractional shortening (FS); and (4) the E-wave transmitral peak velocity
(E-vel) -- that have been associated with survival
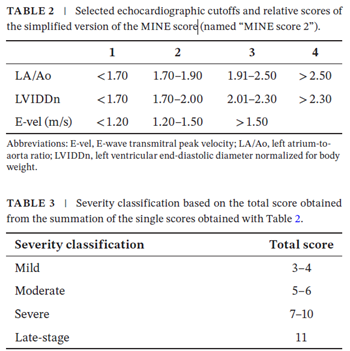 in MVD-affected dogs.
The researchers revised "MINE 2" formula eliminates fractional
shortening (FS) percentage because they have found that FS% "did not
show independent association with cardiac outcomes in the current
investigation. Therefore their simplified "MINE 2" formula is limited to
three measurements -- LA:Ao, LVIDdN, and E-vel. The researchers also
create a sub-category of Stage B2 of MVD, formerly described as "severe"
Stage B2 to now "advanced" Stage B2, meaning being at high risk for
reaching congestive heart failure (CHF). Tables 2 and 3 (at left) show
the researchers' MINE score 2 cut-offs for MVD-affected dogs in Stage
B2. The researchers also suggest that by classifying a severe MINE 2
score, clinical trials using that classification could evaluate such
dogs benefitting from "supplementary treatment in addition to
pimobendan." Presumably they mean adding a diuretic before the dog
reaches CHF.
in MVD-affected dogs.
The researchers revised "MINE 2" formula eliminates fractional
shortening (FS) percentage because they have found that FS% "did not
show independent association with cardiac outcomes in the current
investigation. Therefore their simplified "MINE 2" formula is limited to
three measurements -- LA:Ao, LVIDdN, and E-vel. The researchers also
create a sub-category of Stage B2 of MVD, formerly described as "severe"
Stage B2 to now "advanced" Stage B2, meaning being at high risk for
reaching congestive heart failure (CHF). Tables 2 and 3 (at left) show
the researchers' MINE score 2 cut-offs for MVD-affected dogs in Stage
B2. The researchers also suggest that by classifying a severe MINE 2
score, clinical trials using that classification could evaluate such
dogs benefitting from "supplementary treatment in addition to
pimobendan." Presumably they mean adding a diuretic before the dog
reaches CHF.
RETURN TO TOP
Progression & Prognosis
- Left atrial enlargement
- Left ventricle enlargement
- Increased mitral regurgitation
- Congestive heart failure
- Atrial fibrillation (AF)
- Chordae tendineae rupture
- Left atrial tear
- Atrial septal defects and aneurysms
- Pulmonary hypertension (PH)
- Mitral regurgitation severity index (MRSI)
- Sudden death
 The progression of mitral valve disease can be rapid or slow.
In most breeds, MVD barely progresses beyond a murmur or mild enlargement
for most dogs -- up to 70% to 80% of them. The typical MVD-affected cavalier
King Charles spaniel is not so fortunate. In most CKCSs, the disease shows a gradual progression in the loudness of
the murmur and to more serious symptoms, in as little as two years after
first detecting the murmur. But, some
cavaliers develop a mild murmur without any more serious symptoms for many
years. During this period, the dog's heart is doing its best to compensate
for the affects of the blood backflowing through the valve. However, as
veterinary cardiologist Dr. Stephen Ettinger has observed about the dog's
efforts to compensate for those deliterious affects:
The progression of mitral valve disease can be rapid or slow.
In most breeds, MVD barely progresses beyond a murmur or mild enlargement
for most dogs -- up to 70% to 80% of them. The typical MVD-affected cavalier
King Charles spaniel is not so fortunate. In most CKCSs, the disease shows a gradual progression in the loudness of
the murmur and to more serious symptoms, in as little as two years after
first detecting the murmur. But, some
cavaliers develop a mild murmur without any more serious symptoms for many
years. During this period, the dog's heart is doing its best to compensate
for the affects of the blood backflowing through the valve. However, as
veterinary cardiologist Dr. Stephen Ettinger has observed about the dog's
efforts to compensate for those deliterious affects:
"A common characteristic of all compensatory responses is that the short-term effects are helpful, but the long-term effects are deleterious."
If the progression is slow enough, the dogs may die of other causes before their hearts reach failure. This is the usual pattern of MVD in most other breeds affected with it. Reportedly, up to 80% of small breed dogs which develop MVD murmurs, and even a mild degree of heart enlargement, will not reach Stage C -- congestive heart failure (CHF). But cavaliers are a breed apart in that regard, because the onset of MVD is so early in the CKCS and so pervasive. Therefore, most all cavaliers which develop heart enlargement will reach CHF.
Skipping Stage B2: An MVD-affected dog could have a mild mitral valve murmur and no left atrial enlargement or left ventricle enlargement at all, and then suddenly, that dog could develop heart failure and enter Stage C because one or more of its heart's chordae tendineae rupture.
Once the dog reaches the stage of congestive heart failure (CHF), the average time until death reportedly is 9 months and typically is expected to be between 6 and 14 months. The median survival period for dogs once they develop severe CHF due to MVD is approximately 7 months, with 75% of the dogs dead by one year. For dogs with less severe CHF, the median survival period is one year, with 75% of the dogs dead by 21 months. However, the CKCS has a more accelerated version of MVD, and they typically progress more rapidly to heart failure.
However, in an April 2018 report, a team of researchers at Tufts University's Cummings vet school examined the records of 54 MVD-affected dogs (including 9 cavalier King Charles spaniels) in advanced heart failure (AHF). Their definition of AHF was the recurrence of heart failure signs despite treatment with initial doses of pimobendan, ACE-inhibitor, and furosemide. At the diagnosis of AHF, they found that for most of the 54 dogs, doses of pimobendan, furosemide, ACE-inhibitor, and spironolactone were increased, with new medications added in most dogs. They found that the median survival time after diagnosis of AHF was 281 days (range, 3-885 days), and that dogs receiving a furosemide dose >6.70 mg/kg/day had significantly longer median survival times (402 days [range, 3-885 days] versus 129 days [range 9-853 days]). They concluded that dogs with AHF can have relatively long survival times, and that "a higher furosemide dose was significantly associated with longer survival time."
Risk factors associated with progression of MVD include:
• breed: CKCS is 20 times more prone to have-mitral valve disease than other breeds.
• age
• gender
• grade of heart murmur
• degree of mitral valve regurgitation
• extent of mitral valve prolapse
• severity of mitral valve lesions
• degree of left atrial enlargement
• rupture of chordae tendinae
• pulmonary hypertension
• increased concentration of natriuretic peptides.
Drugs may help to minimize the symptoms of MVD-affected dogs in Stage C, but eventually the drugs may be unable to control them, and they progress to Stage D. Severe symptoms in some cavaliers will appear more quickly, although previously having been stable.
A method which cavalier owners can use to determine if and when their dog reaches the stage of heart failure is to count the dog's breaths per minute while sleeping. Researchers found that healthy adult dogs generally have a "mean" (average) sleeping respiratory rate of less than 30 breaths per minute and rarely exceed that rate at any time. Some cardiologists recommend that their patient's owners periodically count their dog's respiratory rate, and when the average rate starts to creep up to the high twenties, to make an appointment for the dog to be re-examined by the cardiologist to see if the dog is approaching or has reached the stage of heart failure. See our section on Respiratory Rates for more information.
For an in-depth on-line seminar about the symptoms, diagnosis, progression, and treatment of mitral valve disease, watch Dr. Andrew Beardow, with his terrific active graphics, explain MVD.
RETURN TO TOP
• left atrial enlargement
 Enlargement of the
left atrium (LA) results when some of the blood
already pumped from the LA through the mitral valve into the left
ventricle, reverses direction and backflows through the mitral valve
(mitral regurgitation -- MR) back into the LA again. As more blood flows
into the LA from both directions, this volume overload increases stress
on the atrium wall, which in turn drains the heart of its main sources
of energy -- adenosine triphosphate (ATP) and oxygen.
The left atrium necessarily begins to enlarge by stretching, called
dilation. It is classified as Stage B2 in the list of ACVIM stages of
MVD discussed above. (The x-ray at the
right shows
severe enlargement of the left atrium of a dog's heart, outlined in red.)
Enlargement of the
left atrium (LA) results when some of the blood
already pumped from the LA through the mitral valve into the left
ventricle, reverses direction and backflows through the mitral valve
(mitral regurgitation -- MR) back into the LA again. As more blood flows
into the LA from both directions, this volume overload increases stress
on the atrium wall, which in turn drains the heart of its main sources
of energy -- adenosine triphosphate (ATP) and oxygen.
The left atrium necessarily begins to enlarge by stretching, called
dilation. It is classified as Stage B2 in the list of ACVIM stages of
MVD discussed above. (The x-ray at the
right shows
severe enlargement of the left atrium of a dog's heart, outlined in red.)
ATP is essential for the heart to function properly. It is an energy-carrying molecule found in all cells of the body, including in particular, the dog's heart. When energy is needed by the cell, it is converted from storage molecules into ATP. ATP then delivers energy to places within the cell where energy-consuming activities are taking place. When the LA is stressed and over-worked, as it is during volume overload due to mitral valve regurgitation, the process of maintaining necessary levels of the supply of ATP do not meet the demand, and the walls of the LA weaken due to the stress of the volume overload, causing them to stretch and enlarge.
Left atrial enlargement is one of the most reliable predictors of risk factors. In a June 2017 abstract report, the risk of death with a left atrium/aortic root ratio greater than 1.7 was over two times that of dogs with smaller atrial dimensions. In an April 2010 research article, Swedish cardiologists reported finding that cavaliers' left heart chambers increased in size rapidly only during the last year before the onset of heart failure.
RETURN TO TOP
• left ventricle enlargement
The left ventricle (LV) is the main pumping chamber of the heart. As
MVD progresses, the LV may also enlarge.
 But
unlike the enlargement of the walls of the left atrium, which stretch
and thin, the LV walls thicken to increase the force of
its contraction, to compensate for the lessened quantity of blood the LV
is intended to pump though the arteries to the body. This thickening
process is called myocardial hypertrophy. The enlarged LV is
more rounded than a flatter, normal sized LV. It is
classified as Stage B2 in the list of ACVIM stages of MVD
discussed above. (At the left here are
a pair of computer-designed models of a normal sized LV on the
left and an enlarged LV on the right. Note that the healthy LV is
flatter [elliptical], and the enlarged LV is wider and rounded
[globular]). (Image from
Ljungvall, et al. 20111.)
But
unlike the enlargement of the walls of the left atrium, which stretch
and thin, the LV walls thicken to increase the force of
its contraction, to compensate for the lessened quantity of blood the LV
is intended to pump though the arteries to the body. This thickening
process is called myocardial hypertrophy. The enlarged LV is
more rounded than a flatter, normal sized LV. It is
classified as Stage B2 in the list of ACVIM stages of MVD
discussed above. (At the left here are
a pair of computer-designed models of a normal sized LV on the
left and an enlarged LV on the right. Note that the healthy LV is
flatter [elliptical], and the enlarged LV is wider and rounded
[globular]). (Image from
Ljungvall, et al. 20111.)
This enlargement process also saps the LV walls of its energy source, ATP, and the LV also loses the flexibility it needs to act as a pumping device.
In an August 2022 article, Japanese reseachers used echocardiography to measure the mitral valve leaflet-annulus index (LAI) of 83 dogs, including 9 (10.8%) cavaliers, all diagnosed with MVD. The LAI is a complicated calculation which involves taking various measurements of the mitral valve leaflets. The LAI of each dog was compared to other echo measurements, including the left ventricular end-diastolic internal diameter normalized to body weight (LVIDDN), the left atrium to aorta ratio (LA/Ao), and the grade of mitral regurgitation (MR). They observed that the chronic volume overload from MR caused the left ventricle to expand, which in turn caused the mitral annulus to expand, resulting in increased mitral regurgitation.
However, once the MVD-affected dog reaches Stage C and also has been diagnosed with pulmonary hypertension (PH), significant decrease in the size of the LV has been reported. In this December 2023 article, the authors suggest that the reduction in the left ventricular size may be attributed to impaired filling in dogs with PH.
RETURN TO TOP
• increased mitral regurgitation
As the left ventricle enlarges, it has been found to cause the mitral valve to widen and thereby increase the amount of mitral regurgition (MR). Typically, the regurgitating blood shoots back into the left atrium with jet-like force, and eventually may cause damage -- lesions -- to the endothelial cells lining the interior wall of the left atrium. The repetitive jets of blood, with each beat of the heart, may create blood clots, and an atrial septal defect (ASD), in addtion to enlargement of the left atrium. See details about measuring MR above.
In an August 2022 article, Japanese reseachers used echocardiography to measure the mitral valve leaflet-annulus index (LAI) of 83 dogs, including 9 (10.8%) cavaliers, all diagnosed with MVD. The LAI is a complicated calculation which involves taking various measurements of the mitral valve leaflets. The LAI of each dog was compared to other echo measurements, including the left ventricular end-diastolic internal diameter normalized to body weight (LVIDDN), the left atrium to aorta ratio (LA/Ao), and the grade of mitral regurgitation (MR). They observed that the chronic volume overload from MR caused the left ventricle to expand, which in turn caused the mitral annulus to expand, resulting in increased mitral regurgitation.
RETURN TO TOP
• congestive heart failure
Heart failure (HF) has been discussed elsewhere in this article. It is classified as Stage C in the list of ACVIM stages of MVD discussed above. To summarize, HF is the stage of MVD at which HF is a point at which the MVD-affected dog's heart no longer is able to compensate by itself (meaning, without medication) for the debilitating efffects of MVD on the heart's ability to pump adequate quantities of blood throughout the entire body. It is the reduced cardiac output of blood to the body, with signs including exercise intolerance and organ congestion. It is the end result, short of death, of the progression of MVD in the cavalier King Charles spaniel.
"Congestive heart failure" (CHF) refers to the leakage (effusion) of white blood cells through the walls of the capillaries (also called diapedesis) to the surrounding tissues, in this case into the air sacs (alveoli) of the lungs, as a result of increased pressure in the left atrium which then increases pulmonary and venous pressures. The result of this process is called pulmonary edema (oedema).
While CHF is an actual event occuring within the heart and the blood system, it is not detected directly, but instead by outward signs and x-rays.
The first observable indication that the dog is experiencing CHF is an increase in the dog's breaths per minute (tachypnea), called its respiratory rate (RR). This is due to the fluid filling the lungs and stiffening them. When the RR while the dog is at rest (RRR) or preferably while sound asleep (SRR) consistently exceeds 30 to 35 breaths per minute, the dog probably is in congestive heart failure. This is discussed in more detail here.

The CHF-affected dog may not lie down and rather assume a sitting position with spread elbows and an extended neck.
In a March 2020 article, an international team of cardiologists studied 135 MVD-affected dogs, including 62 cavaliers, as they approached and entered CHF. They found:
"Dogs with MMVD in ACVIM stage B2 experience increases in HR [heart rate], RR [clinical respiratory rate], RRR [at-home resting respiratory rate], and VHS [heaert size], and decreases in BW [body weight] and RT [rectal temperature] as they progress into CHF. The change in VHS is characterized by a gradual increase over time, whereas the other variables change within the last few months before CHF. The variables with highest absolute change and rate of change were RR and RRR. This finding reinforces the value of RR and RRR as indicators of impending or incipient CHF. If RR and RRR are routinely and frequently measured in dogs with stage B2 MMVD, it may be possible for the onset of CHF to be detected earlier and therefore for it to be managed sooner and more effectively."
The other observable indication of CHF is fluid in the lungs. X-rays are the best diagnostic device for detecting fluid in the dog's lungs. This is discussed in more detail here. Other typical outward signs of HF or CHF include tiredness, difficulty exercising, fainting (syncope), or collapse (presyncope).
The most important significance of detecting heart failure in the MVD-affected cavalier is that it needs to be treated with a diuretic immediately and most likely for the rest of its life.
RETURN TO TOP
• atrial fibrillation
Atrial fibrillation (AF) is an abnormally rapid and uneven heartbeat. It is attributed to the effects of left artial enlargement (LAE) due to MVD. Not all MVD-affected dogs with LAE develop AF, but most will once they reach heart failure (CHF).
Veterinarians refer to abnormally fast heartbeats (higher than 160 beats-per-minute) as supraventricular tachycardia, and to uneven heartbeats as arrhythmias. So, AF is both a supraventricular tachycardia and an arrhythmia.
AF requires treatment of its own, including digoxin* (Lanoxin, Digitek, Digox, Lanoxicaps, Cardoxin), diltiazem (Dilacor), and lidocaine (lignocaine, Xylocaine), and theophylline** (Corvental, Nuelin, Apo-Theo-LA, Theo-Dur) to control the heart beat, and in more severe situations, sotalol (Betapace, Sorine, Sotylize, Sotamol, Sotacor, Rylosol, Linsotalol), a beta-adrenergic blocker, and amiodarone (Pacerone), a beta-adrenergic and calcium channel blocker. In addition, obesity is one factor associated with AF. The main goal of these medications is to achieve an average heart reate below 125 beats per minute and preferably below 110 beats/minute. Heart rates can be evaluated based on 24-hour Holter ECG monitoring.
*WARNING: Digoxin toxicity (digoxinemia) may occur if the AF patient also has pre-existing ventricular tachyarrhythmias or renal dysfunction, or hpokalemia (low potassium level in blood serum), or in combination with certain other drugs, including diltiazem and spironolactone. See this January 2022 book. See, also, this June 2022 article warning that "life-threatening spikes of digoxinemia could occur, especially after 1-year treatment [for AF] with digoxin." See, also, this August 2022 case report of a dog diagnosed with heart failure and AF and treated with digoxin developed digitalis toxicity and showed a high concentration of plasma digoxin, with these symptoms disappearing 5 days after withdrawal of the digoxin medication.
** Theophylline is a
PDE inhibitor which should not be given concurrently with pimobendan, which
is another PDE inhibitor, unless the combination of those two drugs is
carefully balanced.
In an October 2020 article, researchers examined the veterinary records of 2,194 dogs diagnosed with mitral valve disease (MVD), including only 51 (2.3%) cavaliers. Their objective was to estimate the prevalence of AF in MVD-affected dogs. Only 59 (2.7%) of all of these MVD-affected dogs were diagnosed with AF. They concluded that AF is not commonly associated in MVD-affected dogs, but that left atrial enlargement, increased body weight, decreased fractional shortening (below 40%), and heart failure are risk factors for MVD-affected dogs to develop AF.
RETURN TO TOP
• chordae tendineae rupture
 Chordae
tendineae rupture (CTR) is a special case -- the exception to the
general rule when it comes to predicting the typical progression of MVD.
The chordae tendineae (CT) are the tendons which control the opening and
closing movements of the mitral valve leaflets. They are shaped much
like the chords of a parachute. Some of these chords are small and a few
are quite large. The largest are called "first-order" or major chordae,
and the smaller ones are "second-order" or "third-order" or minor
chordae. See a more detailed explanation of CTs at
this link.
Chordae
tendineae rupture (CTR) is a special case -- the exception to the
general rule when it comes to predicting the typical progression of MVD.
The chordae tendineae (CT) are the tendons which control the opening and
closing movements of the mitral valve leaflets. They are shaped much
like the chords of a parachute. Some of these chords are small and a few
are quite large. The largest are called "first-order" or major chordae,
and the smaller ones are "second-order" or "third-order" or minor
chordae. See a more detailed explanation of CTs at
this link.
As the MVD progresses, these chordae deteriorate in the same manner as the leaflets of the valve. This is attributed to infiltration of chordae with glycosaminoglycans. In some cases, one or more of the chords shred or rupture, and if the ruptured chorda is a large one, or if several of the smaller ones tear apart, the valve leaflet to which the chords had been attached will "flail" or flap as it opens and closes, allowing blood to backflow through the valve at a far more rapid rate than before the rupture.
Thus far, CTR has not been predictable or directly related to the deteriorated condition of the valve flaps. Once a major chorda ruptures, severe signs may appear almost instantly, including cardiogenic shock -- the sudden lack of blood being delivered to the body.
In a 2007 study of 706 MVD-affected dogs, CTR was found to occur in 114 (16.1%) of the dogs, most all of which already had severe mitral regurgitation. While the rupture of chords may quickly send a Stage B2 dog into Stage C -- heart failure -- it does not necessarily mean sudden death for the dog. In that 2007 study, the median survival time for the 114 dogs was 425 days (range 5-1,324 days).
RETURN TO TOP
• left atrial tear
 Tear or rupture
(see image at right) of the wall of the left atrium, usually
the rear (caudal) wall, is a rare but possible consequence of severe MVD. The
left atrium's caudal wall is the location where regurgitant jets of
blood commonly are directed when they backflow through the mitral valve,
causing a trauma to that region of the wall. See these reported cases:
August 2010;
December 2012;
August 2015;
October 2016;
March 2025.
Tear or rupture
(see image at right) of the wall of the left atrium, usually
the rear (caudal) wall, is a rare but possible consequence of severe MVD. The
left atrium's caudal wall is the location where regurgitant jets of
blood commonly are directed when they backflow through the mitral valve,
causing a trauma to that region of the wall. See these reported cases:
August 2010;
December 2012;
August 2015;
October 2016;
March 2025.
As a result of the rupture, fluid may collect in the pericardial sac or pericardium -- the space surrounding the heart. When this fluid leaks through the tear in the left atrium into the pericardium, the condition is called pericardial efffusion or hemopericardium. This accumulation of fluid may compress the heart, leading to a decrease in the heart's output of pumping blood and obstructive shock. This condition often results in sudden death, but not necessarily. See this January 1964 article. In some cases, the left atrial rupture may cause left atrial pressure to decrease, and bleeding may stop and form a clot (thrombus).
If the dog survives, emergency treatment is necessary, which may include medication with atropine, to treat the resulting low heart rate, and dobutamine, a cardiac stimulator. In the event that medication is not successful, a procedure called pericardiocentesis may be performed to remove the fluid from the pericardial sac. See this November 2021 reported case.
In an August 2022 report, three chihuahuas diagnosed with left atrial rupture were surgically treated by suturing the left atrial tear, together with "mitral valvuloplasty" -- which involves mitral valve reconstruction -- including cardiopulmonary bypass. All three patients survived the surgeries, and all MVD medications were withdrawn within the following three months. Read details of mitral valvuloplasty here.
RETURN TO TOP
• atrial septal defects and aneurysms
An atrial septal defect (ASD) is a small hole between the left and
right atria, the two upper chambers of the heart. Most ASDs are the
result of fetal developmental failures. However, cavaliers with advanced
MVD have
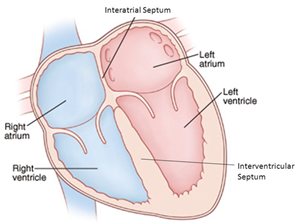
In an August 2010 article, Dr. James Buchanan suggested that spontaneous rupture of the atrial septum may be related to increased left atrial volume and pressure combined with trauma to the atrial wall due to the jet of mitral regurgitation. Endocardial splitting may be preceded by rupture of mitral valve chordae tendinae and genetically influenced variations in endocardial tissue may predispose certain dog breeds to endocardial degeneration and splitting. He recommended that the possible presence of an ASD should be considered in all cases of MVD which also develop signs of right-sided heart failure.
An atrial septal aneurysm (ASA) is a bulging of the septum -- the membrane between the two atria chambers -- from one chamber into the other. An ASA found in an MVD-affected dog diagnosed with severe mitral regurgitation (MR) and increased pressure in the left atrium (LA), may also be associated with an atrial septal defect (ASD).
In an October 2016 article, an MVD-affected cavalier was diagnosed with an atrial septal aneurysm bulging into the right atrium. The dog also had a left-to-right shunt flow through an ASD. The dog died suddenly 2 weeks after being examined.
In an April 2025 article by Italian cardiac clinicians. an 11-year-old cavalier previously diagnosed with Stage B2 MVD developed respiratory distress and abdominal distension. Fluid in the lungs and abdomen (ascites) were diagnosed. An echocardiographic scan revealed an ASD in addition to moderate enlargement of both the left atrium and left ventricle. A blood clot (intracardiac thrombosis -- ICT) within the ASD also was detected. The dog was treated with blood thinning drugs for the clot, in addition to MVD medications. The dog died 138 days after discharge, and the clinicians performed a necropsy (post-mortem examination) which revealed advanced (Type 4) lesions on the mitral valve leaflets, enlargement of the two left heart chambers, several lesions within the left atrium, attributed to jets of blood regurgitating through the mitral valve, extensive damage to the endothelial cells of the left atrium, and the ASD and a blood clot trapped within the ASD. They concluded that the blood clot was triggered by the dog's advanced stage of MVD.
RETURN TO TOP
• pulmonary hypertension
There are two categories of pulmonary hypertension -- high blood pressure in the vessels of the lungs. Their causes usually are dissimilar, so we discuss each of them separately.
pulmonary venous hypertension (PVH)
Increased pressure of the blood in the veins of the lungs is called pulmonary venous hypertension (PVH). PVH in dogs is most often caused by the progression of MVD. Typically, PVH arises as a result of fluid in the lungs (pulmonary edema -- Stage C -- heart failure) and the enlargement of the left atrium and left ventricle of the heart, due to MVD. In turn, long-term PVH can contribute to the continuation of heart failure and also cause the walls of the affected veins to thicken and increase in diameter, which is observable in x-rays.
PH is suspected in dogs if the dogs display symptoms of advanced MVD and certainly of heart failure, including exercise intolerance, excessive panting, coughing, syncope during exertion, respiratory distress while at rest or after exercise, and ascites (fluid in the abdomen or abdominal effusion). Also, significant decrease in the size of the left venricle has been reported in MVD-affected dogs in Stage C as a result of PH. In this December 2023 article, the authors suggest that the reduction in the left ventricular size may be attributed to impaired filling in dogs with PH.
The form of PH most often resulting from MVD is called "post-capillary PH" (as opposed to pre-capillary PH), which is due to backward transmission of the increased filling pressures in the left atrium into the pulmonary circulation. This high pressure may lead to increased right ventricular pressure and right atrial chamber enlargement, leading to possible right side heart failure. Post-capillary PH is not to be medicated with sildenafil (Viagra) or related drugs.
Echocardiography can diagnose PH. High velocity tricuspid regurgitation is an indication of PH, such as over 3 meters per second. A formula is used to calculate the trans-tricuspid gradient.
In a March 2015 study of 212 dogs, including 30 cavaliers, diagnosed with either Stage B2 or Stage C MVD, the researchers found that 39% of the dogs had PH, and that PH was more commonly found in Stage C. They concluded that the presence of moderate to severe PH predicted a shorter survival time in dogs with MVD.
In a June 2019 article, researchers sought to determine if MVD-affected cavaliers are more likely to develop pulmonary hypertension (PH) than MVD-affected dogs of other breeds. Ninety-four CKCSs were compared with 93 other dogs (= 187 dogs) in a variety of stages of MVD, some with PH and some without. They compared several different measurements of MVD, including the ACVIM stages (Stage B2, C, and D), and echo variables of left-atrium-to-aortic ratio (LA/Ao), E wave velocity (E vel) and isovolumic relaxation time (E/IVRT) and found that all of them were significant in the development of PH. However, instead of finding that cavaliers were uniquely disposed to PH, they concluded that for all breeds, the worsening of MVD, from ACVIM stage B2 to C to D, was the predominant determinant of PH development. However, 59.4% of the Stage B2 CKCSs and 38.3% of the Stage B2 non-CKCSs had PH, indicating that the onset of PH can precede the onset of congestive heart failure (CHF). As additional findings, they confirmed that CKCSs are more likely to experience cardiac death than the other breeds, and that PH is associated with greater likelihood of reaching CHF and cardiac death. ("Associated with" means that they cannot confirm that PH is the cause of CHF or death.) They stated that, "Further studies are needed to determine the causative mechanism for PH development in CKCS with MMVD."
In that February 2020 article, the ACVIM has issued a Consensus Statement of guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension (PH) in dogs. They group the PH into six categories, of which Group 2 is PH secondary to left-sided heart disesase, meaning MVD. At the outset for Group 2 PH-affected dogs, they warn that "a PDE5i (e.g. sildenafil, Viagra, Revatio) should be administered only to dogs free of acute or decompensated LHF (cardiogenic pulmonary edema). In other words, if the MVD-affected dog is in Stage C (heart failure) and still has fluid in its lungs, treating with Viagra is not appropriate. They add that PDE5i drugs may be considered if the dog has exertional syncope due to its MVD, and it has failed to respond to other treatments, specifically pimobendan.
In a June 2022 article, Thai researchers outlined the factors relating to the survival time of MVD-affected dogs also diagnosed with PH. The results showed that the median survival time of dogs with PH due to Stage C of MVD was 368 days, and factors that shortened the median survival time of dogs with PH were the presence of right heart enlargement, ascites and high probability of PH. Factors associated with an increased hazard of death were right heart enlargement and ascites.
In a September 2022 article, South Korean researchers examined the x-rays of the hearts of 302 dogs diagnosed by echocardiography to have mitral regurgitation (MR). They compared the diameters of the cranial and (upper) caudal (lower) pulmonary arteries and veins to the fourth rib and the ninth rib, and they calculated the ratio of the pulmonary artery to the corresponding vein (CdPA/CdPV). They diagnosed pulmonary hypertension was diagnosed in 77 of the dogs (25.5%) and found that the prevalence of PH increased with the severity of MR. They reported finding that the CdPA/CdPV was "significantly higher in the presence of PH". The cut-off value of the CdPA/CdPV = 1.10 showed 90.6% specificity and 31.1% sensitivity for detecting PH in dogs with MR. They concluded that, in dogs with MR, PH can be predicted with high specificity when the caudal pulmonary artery is 1.1 times larger than the corresponding vein on x-rays.
RETURN TO TOP
pulmonary arterial hypertension (PAH)
Pulmonary hypertension (pulmonary arterial hypertension -- PH or PAH) is the name given to increased blood pressure in the pulmonary (lungs) arteries, which lead from the heart to the lungs. While PH can have various underlying causes*, the most common one is a complication of mitral valve disease, usually in Stage C. The prevalence of PH in dogs in Stage C of MVD has been reported in the range of 40+%. See, e.g., this December 2023 article.
* Other causes include drug reactions, toxins, parasites, primary lung disease, upper airway obstruction, high altitude, blood clot, heartworm, brachycephalia, other cardiac diseases, pneumonia, Cushing's disease, immune-mediated hemolytic anemia, neoplasia, and idiopathic.
PH is higher diastolic or systolic pulmonary arterial pressure than normal pressure. Normal pulmonary arterial pressure is 10-15 mm Hg (mercury), and PH is defined as a sustained pressure greater than 25 to 30 mm Hg at rest. Less than 40 mm Hg is considered "mild" PH. Between 40 and 80 mm Hg is "moderate" PH, and higher than 80 mm Hg is "severe". Pressure at or above 48 mm Hg suggests that the PH is irreversible. In a February 2020 ACVIM Consensus Statement on PH, the authors recommend defining PH in dogs as having a tricuspid regurgitation pressure gradient cutoff of >46 mmHg (TRV >3.4 m/s).
RETURN TO TOP
• mitral regurgitation severity index (MRSI)
The "Mitral Regurgitation Severity Index" (MRSI) is a somewhat complex index of data obtained from the MVD-affected dog, which includes the dog's heart rate, its echocardiographic left atrium-to-aorta ratio (LA/Ao), and its age. It has been devised by Dr. Claude E. Atkins, board certified veterinary cardiologist at North Carolina State University (right). The announced purpose of the MRSI is to index the likelihood of MVD-affected dogs in Stage B2 reaching heart failure. Its formula includes three variables -- age, heart rate, and echocardiographic left atrium-to-aorta ratio (LA/Ao) -- normalized based upon average values derived from the VETPROOF* study dataset, to arrive at a specific three-digit number within a range from less than 150 to above 249. Specifically, the equation is:
MRSI = (heart rate/120) x LA/Ao x (dog's age/10) x 100
Theoretically, the lower the MRSI for MVD-affected dogs in Stage B2, the less severe the MVD and the longer the number of days before the dog reaches Stage C, heart failure, if at all. In a June 2022 abstract, Dr. Atkins and others (Darcy Adin, Thomas Blondell, Emilie Guillot, Michelle Vereb, and Jessica Ward) reported on the application of the MRSI to 133 dogs diagnosed with Stage B2 of MVDThey form the title of their report as a question: "Mitral Regurgita tion Severity Index: A Simple Calculation for Classification and Prognostication?". (Does this mean he is not sure?) The authors arrived at three "severity indices" of the MRSI, as follows:
• MRSI below 150: 981 median days to CHF (range: 444 days to NA)
• MRSI between 150 and 249: 587 median days to CHF (range: 369 to 907 days)
• MRSI above 249: 307 median days to CHF (range: 99 to 734 days).
* VETPROOF (Veterinary Enalapril Trial to Prove Reduction in Onset Of heart Failure) was a 139 MVD-affected dog study reported in 2002, which followed the dogs from the time in which they were diagnosed with severe MVD but without heart failure until the onset of heart failure. From these data, prognostic factors (historical, physical, radiographic, and echocardiographic variables) were determined.
RETURN TO TOP
• sudden death
MVD-affected dogs may die suddenly and without much or any preliminary indications, due to any of these causes listed above: atrial fibrillation, chordae tendineae rupture, atrial tear (rupture).
RETURN TO TOP
Treatment other than medication
- Dietary treatment
- Supplements
- Supplements to avoid
- Stem cells
- Growth differentiation factor 11 (GDF11)
- New DNA injections
- Inflammation treatments
- Physical exercise
It is unrealistic to try to cure canine mitral valve disease. "Management" is a word frequently used by veterinary cardiologists to describe the conventional means of treating MVD. It involves medications, supplements, and diets intended to compensate for the progression and symptoms of MVD, especially once the disease reaches heart failure (HF). The veterinarian tries to eliminate or reduce signs of fluid accumulation and congestion, and to maintain adequate cardiac output in order to provide needed blood flow. The degree of treatment will depend upon the stage of the disease. Early MVD is not treated in the same way as advanced MVD. The particular management treatments are discussed below: Stage B1 (mild), Stage B2 (moderate), Stage C (severe), or Stage D (end stage).
For an in-depth on-line seminar about the symptoms, diagnosis, progression, and treatment of mitral valve disease, watch Dr. Andrew Beardow, with his terrific active graphics, explain MVD.
RETURN TO TOP
-- dietary treatment
General nutrition
 General
nutrition for dogs diagnosed with mitral valve disease (MVD) is very important as
the MVD progresses
through its stages. As MVD worsens, the dog's loss of skeletal muscle
mass (cardiac cachexia) is a major threat to its survival. See our section on exercise
intolerance and loss of skeletal mass for details about cardiac
cachexia.
General
nutrition for dogs diagnosed with mitral valve disease (MVD) is very important as
the MVD progresses
through its stages. As MVD worsens, the dog's loss of skeletal muscle
mass (cardiac cachexia) is a major threat to its survival. See our section on exercise
intolerance and loss of skeletal mass for details about cardiac
cachexia.
MVD-affected dogs need complete proteins from animal sources (muscle meats from mammals, poultry, fish, eggs), which provide all of the essential amino acids. Plant-based foods which may provide some proteins, are not sufficiently healthful alone for dogs diagnosed with MVD.
All meats and vegetables should be as fresh and un-processed as possible. The MVD-affected dog's food should not be overly processed, such as dry dog foods (kibble) are, because each step in that processing removes natural nutrients esssential for a complete, well balanced diet. Meats should be changed periodically, such as each month, to assure that the dog is ingesting nutrients from a variety of sources.
Grains may be included or not, depending upon the other health issues of the dog, but grains never should be relied upon as the main sources of proteins for MVD-affected dogs. Diets containing grains and animal by-products have more limited digestibility and poorer essential amino acid balance, than do meats that are not heavily processed. See this January 2026 article.
Before you buy any dog food, read the list of ingredients on the container. Or, better yet, find the ingredients list on-line before going to the store. Chewy.com is a good on-line source for finding ingredients lists of the many brands of dog food they offer for sale. Look for "Nutritional Information" on each food's webpage.
The ingredients are listed in descending order by weight on those lists. So, the first ingredient is the heaviest and the last ingredient is the lightest. If the first ingredient is an identifiable muscle meat, such as "beef" or "turkey", that is a positive piece of information about that food. But, for example, if the second through fifth ingredients are grains, such as corn, wheat, soy, then very likely the combination of weights of those grains far outweigh the quantity of meat listed first. And, of course, if the meat is listed as "by-product", that means it is not muscle meat at all.
Generally speaking, the fewer items in an ingredients list means the less processed the food is and the fewer additives there are in that food. That is especially the case if the items low on the list are oddly named and not identifiable as vegetables or fruits. Artificial forms of vitamins and other nutrients are added to foods if the natural sources of those nutrients were destroyed in the processing (or not existing to begin with).
For example, if the amino acid taurine is listed separately way down on the list, that likely means that the muscle meat (if any) was so overly processed that the natural taurine in that meat has been destroyed, so the maker had to add artificial taurine to the ingredients to make up for the destruction of the natural taurine in the muscle meat. Dogs produce taurine in the liver of their own bodies. As long as MVD-affected dogs are fed sufficient fresh muscle meats in their diets, they should not need chemically-produced supplemental taurine unless their blood tests show a taurine deficiency. See this link for more information about taurine supplementation.
 An example of a truly unhealthful food for MVD-affected cavaliers to
avoid is Royal Canin's "Cavalier King Charles Adult Dry Dog Food".
According to
Royal Canin, this food's major ingredients are:
An example of a truly unhealthful food for MVD-affected cavaliers to
avoid is Royal Canin's "Cavalier King Charles Adult Dry Dog Food".
According to
Royal Canin, this food's major ingredients are:
"Brewers rice, wheat gluten, chicken by-product meal*, corn, chicken fat, wheat, natural flavors, dried plain beet pulp, fish oil, pea fiber, dried tomato pomace, vegetable oil, rice hulls".
Notice that there is no muscle meat included in this list, at all. The sources of protein are grains -- rice, wheat, corn. "Chicken by-product meal" by definition* does not include muscle meat. So, this food is not providing the MVD-affected cavalier with all of the essential proteins from natural sources, which are necessary for the dog to maintain as healthy a heart as possible.
See also our section below on so-called "prescription diets" to avoid.
* The Association of American Food Control Officials (AAFCO) defines "poultry by-products" as: "non-rendered clean parts of carcasses of slaughtered poultry, such as heads, feet and viscera, free from fecal content and foreign matter except in such trace amounts as might occur unavoidably in good factory practice. If the product bears a name descriptive of its kind, it must correspond thereto."
RETURN TO TOP
MVD-affected dogs need sodium:
 Unlike humans with heart conditions, who are on strict low or no sodium
diets, MVD-affected dogs need
sodium (table salt -- sodium chloride) to offset the effects which both MVD and its
medications, especially loop diuretics, like furosemide (Lasix) and
torsemide, have upon both the heart and the kidneys. These diuretics
drain water from the body, and so they are a main medication for drawing
fluids from the lungs of MVD-affected dogs in Stages C and D (congestive
heart failure). While that process is good for the heart and lungs, it
unfortunately irritates the kidneys to no end. Also, an excessively low
sodium level is an electrolyte disorder called hyponatremia. See also
hypochloremia (discussed below), an
excessively low chloride level, resulting from both MVD and loop
diuretics.
Unlike humans with heart conditions, who are on strict low or no sodium
diets, MVD-affected dogs need
sodium (table salt -- sodium chloride) to offset the effects which both MVD and its
medications, especially loop diuretics, like furosemide (Lasix) and
torsemide, have upon both the heart and the kidneys. These diuretics
drain water from the body, and so they are a main medication for drawing
fluids from the lungs of MVD-affected dogs in Stages C and D (congestive
heart failure). While that process is good for the heart and lungs, it
unfortunately irritates the kidneys to no end. Also, an excessively low
sodium level is an electrolyte disorder called hyponatremia. See also
hypochloremia (discussed below), an
excessively low chloride level, resulting from both MVD and loop
diuretics.
Dogs' kidneys operate most effectively with normal amounts of water and sodium flowing through the blood stream. When the kidneys detect dehydration and/or low levels of sodium in the blood, they release renin, a combination of amino acid residues which form a peptide, into the bloodstream. This renin triggers a cascade of peptides ("angiotensin I and II"), followed by the "angiotensin converting enzyme (ACE)", and then the hormone "aldosterone", which when combined is called the "renin-angiotensin-aldosterone system" (RAAS). This RAAS acts to narrow the blood vessels, increase blood pressure, and conserve sodium. The RAAS also acts upon the brain, causing the dog a sense of increased thirst and an appetite for salt.
Narrowing of blood vessels and high blood pressure are the last two things any MVD-affected dog needs to have happen. Indeed, heart medications such as pimobendan (Vetmedin, Pimomedin) and sildenafil (Viagra) are among the MVD drugs designed to widen the blood vessels and lower the blood pressure. Benazepril and enalapril are angiotensin converting enzyme inhibitors (ACE-I), having the main purpose of offsetting the effects of the activated RAAS.
Activation of the RAAS also has been identified as either causing or aggravating chronic kidney disease (CKD), excessively high blood pressure, and proteinuria (excess of proteins in the blood).
In the ACVIM's 2019 Consensus Statement, that panel of cardiologists recommends only "modestly" restricting sodium intake. Specifically, they state:
"Modestly restrict sodium intake, taking into consideration sodium from all dietary sources (including dog food, treats, table food, and foods used to administer medications) and avoid any processed or other salted foods." (Emphasis added.)
In a January 2017 article, Dr. Anton C. Beynen reviewed sodium restricted diets for MVD-affected dogs and concluded:
"There is no evidence that sodium restriction improves clinical signs in canine cardiac disease. Worse still, there are good reasons for contraindication."
In the 2012 book, Applied Veterinary Clinical Nutrition, the authors of the chapter, "Nutritional Management of Cardiovascular Diseases", state this about sodium in dog foods:
"The sympathetic nervous system and the RAA system become increasingly activated as heart disease progresses. Thus, severe sodium restriction in animals with early heart disease could theoretically be detrimental by early and excessive activation of the RAA system. The results of one study reported that a low-sodium diet fed to dogs with asymptomatic CVD [cardiac valvular disease] resulted in increased aldosterone concentrations and heart rate, with no improvement in cardiac size or function. Because of the potential detrimental effects and lack of documented benefits of severe sodium restriction in asymptomatic disease, the authors recommend only mild sodium restriction (<100 mg/100 kcal) in asymptomatic heart disease (ISACHC Stages 1a and 1b) [Stages B1 and B2]."
"In dogs with ISACHC Stage 2 [late Stage B2 or early Stage C], the authors recommend moderate sodium restriction (i.e., <80 mg/100 kcal)."
Veterinary cardiology researchers feed low sodium dog foods to healthy dogs to intentionally activate their RAAS in order to test the effectiveness of ACE-inhibitor medications. For example, in a July 2022 article, Iowa State Univ. researchers fed nine healthy dogs a low-sodium diet of Hill's Prescription Diet h/d dry food for five days. Their levels of sodium reached such low levels that it resulted in steady activiation of the dogs' renin-angiotensin-aldosterone system (RAAS). The researchers intentionally wanted to activate the RAAS in order to conduct a study of dosages of the ACE-inhibitor benazepril. In a November 2024 article, researchers testing Cardalis (benazepril/spironolactone) on the renin-angiotensin-aldosterone system in healthy dogs intentionally activated their RAAS system by feeding them the low-sodium diet of Hill's Prescription h/d Heart Care.
MVD-affected cavaliers should avoid low sodium diets, such as Hill's Prescription Diet h/d dry food (with only 17 mg sodium per 100 kcal, 0.12%). To avoid activation of the RAAS, the minimum amount of sodium should never be below the range of from 22.5 to 62 mg per 100 kcal. A solution to the question of what to feed the MVD-affected cavalier, is to choose a high-quality canned or frozen food with fresh, identifiable meats (for example, beef, turkey, chicken -- not "poultry" or "meat") as the main sources of protein and with a moderate amount of sodium (but not a low level), and avoid high sodium dry foods and treats.
The International Small Animal Cardiac Health Council (ISACHC) recommends these minimum amounts of sodium in the diets of MVD-affected dogs:
• Stage B1 or B2: no less than 100 mg/100 kcal.
• Stage C: no less than 80 mg/100 kcal
• Stage D: no less than 50 mg/100 kcal
Hypochloremia:MVD-affected dogs also may need chloride supplementation. Loop diuretics administered to dogs in congestive heart failure -- Stages C and D of MVD -- may result in low levels of chloride in the blood, a serious disorder called hypochloremia. See this January 2021 article. As with low sodium levels, hypochloremia is associated with activation of the kidneys' RAAS. When table salt (sodium chloride) is significantly reduced or even eliminated in the MVD-affected dog's daily diet. the level of chloride is reduced proportionately to the reduction of sodium.
In an October 2025 article which examined 292 dogs in CHF, including 16 cavaliers, the researchers found that moderate to severe hypochloremia is associated with shortened survival in CHF dogs. Depending upon the severity of the hypochloremia, an increase in table salt (sodium chloride) consumption may be prescribed.
RETURN TO TOP
"Prescription Diets" to avoid:
Beware of dog foods touted by veterinarians as "prescription diets" which are claimed to be designed to treat dogs with heart problems. Some vets, indeed many of them, fall for any dog food with the word "cardiac" on its label and which they "prescribe" for MVD-affected dogs. Here are two of the absolute worst such foods:
• Hill's Heart Care h/d: This dry food has an excessively low quantity of sodium, which has been known to activate the dog's kidneys' RAAS. In a July 2022 article, researchers fed healthy dogs this food for five days. Their levels of sodium reached such low levels that it caused the dogs' RAAS to steadily activate. In a November 2024 article, researchers testing Cardalis (benazepril/spironolactone) on the renin-angiotensin-aldosterone system in healthy dogs intentionally activated their RAAS system by feeding them the low-sodium diet of Hill's Prescription h/d Heart Care.
• Purina Pro Plan CC Cardiocare: This dry food has been found in a peer-reviewed study to have no significant effects upon the left atrium and left ventricle in 101 MVD-affected dogs (29 CKCSs). It also conains medium chain triglycerides, which have been found to be dangerous to a high percentage of cavaliers. See below.
See more about these two hazardous-to-cavaliers dog foods at this link.
RETURN TO TOP
Cardiac Cachexia:
Cardiac cachexia is the loss of lean muscle mass, especially in the hind quarters, which has been found primarily in MVD-affected dogs in congestive heart failure (CHF). The loss of body mass in these dogs has deleterious effects upon the dogs' strength and immune functions. For more information about cardiac cachexia, see our section above on the exercise intolerance and loss of muscle mass in the Symptoms section.
In a May 2008 article associating different body weights and body conditions with survival of dogs in heart failure, the researchers found that dogs classified as overweight but not obese had the longest survival rate, compared to the other categories (emaciated, underweight, ideal weight, and obese).
Proteins are the most important sources of nutrition for dogs diagnosed with MVD, and especially those in Stage C, heart failure. Biodegradable proteins, meaning primarily from animal muscle meats, should not be restricted in any diet for MVD-affected dogs. The minimum maintenance requirement for protein is 51 grams per 100 kcal.
RETURN TO TOP
 -- supplements
-- supplements
- In General
- CoQ10
- D-ribose
- Omega-3 fatty acids
- hawthorn
- Magnesium
- Salvia Shou Wu
- Wu Ling San
- Supplements to avoid
- Taurine
- L-carnitine
- Medium chain triglycerides (MCTs)
- Problems with compound heart supplements
MVD-affected dogs will need their diets fortified with heart supplements. Some supplements will benefit the dog in general. Others are mostly beneficial once MVD has been diagnosed. Others should be held back, or not increased in dosages, until the stage of MVD is more advanced than just having a murmur. Most heart supplements are compatible with heart medicines, and in some instances, the supplements will complement the drugs to the extent that lower doses of the drugs may be possible.
From the beginning, even prior to a murmur being detected, a good multi-vitamin, Omega-3s, and a low dose of CoQ10 should be beneficial. Others to add after MVD is diagnosed and progresses are d-Ribose, hawthorn, magnesium, and a higher dose of CoQ10.
--- In General
Preventative Vitamins and Supplements?
No medications or food supplements are known to prevent the onset of MVD. However, some supplements may defer the time of onset (although there is no scientific proof that they do so), including:
Vitamin C (300 to 400 mg. daily)
Vitamin E -- Tocopherol (100 I.U. daily)
CoQ10 (100 mg. twice daily, for small dogs)
Ribose or d-Ribose (see this dosage chart)
Fish oils high in Omega 3 (about 400 mg. daily). as in canned sardines
Antiox-Ultra 5000 by Sogeval Laboratories (a nice all-in-one blend of antioxidants in a chewable tablet.)
• A good multi-vitamin, like VetriScience Canine Plus MultiVitamin, which provides vitamins C, D, and E.
• Fish oils for Omega-3s. Consider adding about an ounce of canned sardines to 3 or 4 meals per week, as a natural source of Omega-3s.
• Vetri-Science Cell Advance 880 Immune Health Antioxidant Support.
• CoQ10 (read about it below).
• D-Ribose (read about it below).
• Arjuna
• Standard Process Canine Cardiac Support (the only compound heart supplement we recommend).
Vitamins and food supplements such as these may be prescribed for all stages of mitral valve disease. Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
RETURN TO TOP
--- CoQ10
Bottom Line: The most recently published studies of CoQ10 for MVD-affected dogs is that: A daily CoQ10 dose of 200 mg [of ubiquinone] (a) was sufficient to achieve at least a 3-fold increase in plasma CoQ10 concentration and (b) may indicate the anti-inflammatory role of CoQ10 in systemic inflammation in dogs with CHF due to MVD.
In general:
 Coenzyme Q10
(CoQ10 or
ubiquinone or
ubiquinol) has been reported to be of benefit and is
recommended as a nutraceutical in human heart failure (CHF) patients.
Most recently, in a
July 2022 article, a team of Slovenian researchers studied the effects of CoQ10 supplementation (100 mg of
ubiquinone twice daily) for three months on dogs diagnosed with either
Stage B2 or Stage C of MVD, compared to placebo
groups of Stage B2 and Stage C dogs and a group of healthy control dogs.
This is the longest term study of CoQ10 in MVD-affected dogs, to date.
They report these findings:
Coenzyme Q10
(CoQ10 or
ubiquinone or
ubiquinol) has been reported to be of benefit and is
recommended as a nutraceutical in human heart failure (CHF) patients.
Most recently, in a
July 2022 article, a team of Slovenian researchers studied the effects of CoQ10 supplementation (100 mg of
ubiquinone twice daily) for three months on dogs diagnosed with either
Stage B2 or Stage C of MVD, compared to placebo
groups of Stage B2 and Stage C dogs and a group of healthy control dogs.
This is the longest term study of CoQ10 in MVD-affected dogs, to date.
They report these findings:
• Plasma CoQ10 concentrations were significantly higher in the CoQ10 groups of Stages B2 and C dogs than in the placebo groups and healthy dogs.
• Doses of 100 mg twice per day significantly increased plasma CoQ10 concentration in both CoQ10 groups (Stage B2 and CHF) compared to the placebo groups.
• A significant increase in plasma CoQ10 concentration was observed in the CoQ10 groups compared to the placebo groups.
• No significant adverse effects were noticed during the study.
• No positive effects of CoQ10 on echocardiographic parameters were detected.
• Only neutrophil percentage and lymphocyte percentage and concentration in CHF dogs were positively affected by supplementation.
They concluded that the positive effect of CoQ10 supplementation -- decreasing the neutrophil percentage and increasing the lymphocyte percentages in CHF dogs -- may indicate the anti-inflammatory role of CoQ10 in systemic inflammation in dogs with CHF due to MVD.
In a 2000 experimental study, the antioxidant CoQ10 reportedly had some limited success in treating dogs in heart failure. It aided the left ventricle in properly relaxing and filling with blood properly.
More favorably, in a September 2018 article, Thai veterinary cardiologists tested 13 dogs in congestive heart failure (CHF) due to MVD with CoQ10 (the ubiquinone formulation) over a four-week period. The dose was 100/mg twice a day for both small (Group I) and large dogs (Group II). However, these researchers concluded by recommending "supplementation of CoQ10 in dogs should be based on body weight." So, that would amount roughly to 5 mg per pound of body weight, twice a day. They report finding that:
• CoQ10 caused a reduction of cardiac troponin I (cTnI) level in 71% of the dogs.
• Systolic function -- fractional shortening (FS) and ejection fraction (EF) -- increased significantly in small dogs after CoQ10 supplementation.
• Other echocardiographic parameters were not altered in either group.
• CoQ10 did not alter sympathovagal balance in MMVD dogs.
• Supplementation dose of CoQ10 should be based on the body weight.
In a June 2020 abstract, Slovenian researchers tested daily doses of water-soluable CoQ10 (ubiquinone) supplementation in 19 MVD-affected dogs in CHF for two weeks, and compared them to 12 healthy dogs. They divided the MVD-dogs in three groups and dosed 100 mg, 200 mg, or placebo divided into two doses each day. One week after the last dose, the CoQ10 concentrations had decreased, but in the 200 mg group, it remained significately higher. They concluded that:
"A daily dose of 200 mg resulted in at least a 3-fold increase of plasma CoQ10 concentration in all patients and might be used in supplementation studies in canine CHF patients."
In a September 2020 article, a team of Danish investigators conducted a 3-week study of 18 cavaliers diagnosed with MVD in either Stage B2 or Stage C, to determine the pharmacokinetics* of repeated oral dosing of the ubiquinone form of CoQ10 and to evaluate echocardiographic parameters, circulating cardiac biomarkers, and quality of life (QoL) after treatment. The dose was 100 mg given orally twice a day in a placebo-controlled trial. They found that a 3-week period of treatment with CoQ10 did not significantly improve echocardiographic indices of MVD severity, circulating cardiac biomarkers, or owner perceived QoL, compared to the placebo. The concluded:
"In conclusion, we show that a gelatin capsule formulation of solubilized Q10 is well tolerated in CKCS with MMVD and is absorbed into the circulation without adverse reactions. There were, however, inter-individual variations in the plasma concentrations with this formulation of Q10 similarly to findings in humans. A dosage of 200 mg/day for three weeks of Q10 does not appear to change the clinical severity of MMVD or QoL in CKCS. The estimated T1/2 of 2.95 days may suggest that every other day treatment in dogs may su ce to keep circulating concentrations of Q10 > 2.0 μg/mL in dogs. The variation in Q10 plasma concentrations observed among the CKCS suggests initiation of studies investigating the physiological factors determining intestinal absorption of Q10, including dosing and feeding regimens. ...Ultimately, investigation of long-term use of Q10 in dogs with MMVD and heart diseases of other etiologies are therefore relevant -- preferably with dosing tailored in accordance with laboratory testing of Q10 plasma concentrations."
* Pharmacokinetics is the study of the movement of drugs in the body, including the processes of absorption, distribution, localization in tissues, biotransformation, and excretion.
In an April 2021 article, a team of researchers from Slovenia tested 18 dogs in heart failure due to MVD, including one cavalier, and 12 healthy dogs in the placebo group, to determine the dose of coenzyme Q10 (ubiquinone) needed to reach a 3-fold increase in plasma CoQ10 concentration in the MVD-affected dogs. The dogs were given daily doses of either 50 mg or 100 mg of ubiquinone twice a day for two weeks and had their plasma CoQ10 concentrations measured at various times. They found that the change in plasma CoQ10 concentration after supplementation began was significantly higher than in the placebo group at 4 hours and 1 and 2 weeks for dogs in the 200-mg group and at 1 and 2 weeks for dogs in the 100-mg group. They concluded that a daily CoQ10 dose of 200 mg was sufficient to achieve at least a 3-fold increase in plasma CoQ10 concentration and may be used in CoQ10 supplementation studies involving dogs with CHF due to MMVD.
RETURN TO TOP
Ubiquinone versus ubiquinol:
Bottom Line: Regardless of the form of the CoQ10 (ubiquinone or ubiquinol) taken, once it is ingested by the dog, it is converted back and forth between ubiquinone and ubiquinol as the dog's body needs it.
 First, to be specific, the term CoQ10 is used
for ubiquinone (fully oxidized state), and CoQ10H2 is
used for ubiquinol (fully reduced state). Ubiquinone
has been the subject of many research projects in humans and a few in dogs.
Ubiqinol, on the other hand, has been the subject of few published
studies in humans and none in dogs. Ubiquinone is a supplement which
produces known benefits, particularly in its role as an ingredient in
the body's mitochondria, which create energy-rich molecules in the
cells, and essential for the production of adenosine triphosphate (ATP)
energy to support the heart.
First, to be specific, the term CoQ10 is used
for ubiquinone (fully oxidized state), and CoQ10H2 is
used for ubiquinol (fully reduced state). Ubiquinone
has been the subject of many research projects in humans and a few in dogs.
Ubiqinol, on the other hand, has been the subject of few published
studies in humans and none in dogs. Ubiquinone is a supplement which
produces known benefits, particularly in its role as an ingredient in
the body's mitochondria, which create energy-rich molecules in the
cells, and essential for the production of adenosine triphosphate (ATP)
energy to support the heart.
In a January 2019 article which examined a variety for formulations of both CoQ10 versions in 14 young, healthy humans, the researchers reached the conclusion that, regardless of which of the two versions were administered, it appeared that the method used to prepare the CoQ10 was most determinative of the degree of bioavailability of the CoQ10 in the human body. They tested six formulations of ubiquinone and one of ubiquinol and found that Pharma Nord's Myoqinon formualtion of ubiquinone (a patented soft gel, soy oil matrix, drug specification heat/cooling recrystallization procedure) was the most effective.
Most recently, in a July 2022 article, the effects of 100 mg of ubiquinone twice daily for three months on dogs diagnosed with either Stage B2 or Stage C of MVD, decreased the neutrophil percentage and increased the lymphocyte percentages in CHF dogs, which may indicate the anti-inflammatory role of CoQ10 in systemic inflammation in dogs with CHF due to MVD.
Ubiquinol is a reduced form of ubiquinone. An October 2021 study of dogs reported finding that, regardless of whether CoQ10 is ingested as either ubiquinone or ubiquinol, it is converted back and forth between the two states as it passes through the digestive system and the bloodstream. For example, Substantially all CoQ10 taken as ubiquinol is oxidized into the ubiquinone state in the stomach and intestines. Then, when it passes from the intestines to the lymph system, it converts back to ubiquinol and most of it remains in that state as it enters the blood system. This author published further reasearch comparing the two versions in a December 2021 article, in which he concluded:
"Based on the data from the lab study and the large dog study, we concluded that ubiquinol in commercial nutritional supplements will most likely be oxidized to ubiquinone before it reaches the absorption cells and that the Coenzyme Q10 in the ubiquinol supplements will be absorbed predominantly in the ubiquinone state, transfer into the lymph nodes predominantly in the ubiquinone state and be reduced back to ubiquinol in the lymphatic system."
Ubiquinone in its fluid state is orange in color. Ubiquinol is milky white but will turn to orange once exposed to the air, as it oxidizes and becomes ubiquinone.
RETURN TO TOP
--- D-ribose
Bottom Line: D-ribose (ribose) is recommended as a twice daily supplement with food for dogs diagnosed with mitral valve disease.
 D-ribose
as a nutritional supplement provides needed added energy to the heart
muscle and walls. It is a natural sugar which the canine body produces
on its own but which can be depleted when the stressful forces of MVD
begin to drain the heart of its ability to compensate for the affects of
mitral valve regurgitation. The dog's heart and skeletal muscles can
only make enough D-ribose to manage their day-to-day needs when those
cells are not under stress. When dogs are in heart failure or are
approaching it, the heart cells cannot make enough D-ribose.
D-ribose
as a nutritional supplement provides needed added energy to the heart
muscle and walls. It is a natural sugar which the canine body produces
on its own but which can be depleted when the stressful forces of MVD
begin to drain the heart of its ability to compensate for the affects of
mitral valve regurgitation. The dog's heart and skeletal muscles can
only make enough D-ribose to manage their day-to-day needs when those
cells are not under stress. When dogs are in heart failure or are
approaching it, the heart cells cannot make enough D-ribose.
Literally, heart failure is an "energy-starved heart." D-ribose is a primary component of adenosine triphosphate (ATP), an energy-carrying molecule found in the cells of all living things. ATP is the means of transforming the energy from the breakdown of food molecules into the energy consumed by all cells of the body, including in particular, the dog's heart. ATP is essential for the heart to function properly. D-ribose is the only compound used by the dog's body to replenish diminished ATP energy stores.
When the MVD-affected left atrium (LA) and left ventricle (LV) of the heart are stressed and over-worked, as they are during volume overload due to mitral valve regurgitation, the heart consumes greater quantities of ATP. At that point, the process of the heart maintaining necessary levels of ATP does not meet the demand, and the walls of the LA and LV weaken due to the stress of the volume overload, causing them to stretch and enlarge. The dog's natural replenishment of necessary levels of ATP can take a considerable amount of time in the hearts of dogs affected with MVD. D-ribose, when taken as a nutritional supplement, bypasses the slow conversion steps needed to recreate ATP and rapidly replenishes the supply of ATP which the heart needs to offset the stresses of mitral regurgitation.
D-ribose is available on-line and at health food stores. All holistic supplements should be taken only if advised to do so by an holistically trained, licensed veterinarian. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
Single doses significantly higher than the recommended dosages may cause mild gastro-intestinal discomfort or transient lightheadedness. D-ribose shoud be given with meals. In diabetic dogs prone to hypoglycemia, it is recommended that D-ribose be given mixed with a fruit juice.
See these articles for more information:
• February 1989: "No significant ATP recovery occurred after 24 hr in the control dogs, but in the ribose-treated animals, ATP levels rebounded to 85% of control [initial values] by 24 hr. Total myocardial adenine nucleotide content and energy charge also recovered in the ribose group but not in the control animals. The ribose infusion, therefore, significantly enhanced the recovery of energy levels in the postischemic myocardium in the intact animals."
• Summer 2004: "Patients with congestive heart failure often experience fatigue despite intensive pharmacological therapy. Ribose can aid the recovery of ATP levels and, hence, diastolic function. Clinical trials have shown that ribose supplementation improves ischemic threshold and enhances diastolic function in congestive heart failure."
• July 2007: "We then turned our attention to ribose. Ribose is made in your body in a slow, laborious process and cannot be found in food. ... Not having ribose would be like trying to build a fire without kindling--nothing would happen. ... I am now recommending ribose for ... heart problem patients ... . In fact, one patient in our study had atrial fibrillation that resolved with ribose, and most practitioners using it are finding it to be simply outstanding for their cardiac patients in general."
• May 2009: "The preservation of ATP is vital in the heart as a reduction in ATP level corresponds to a loss of diastolic function. The administration of ribose supports purine synthesis and salvage pathways promoting diastolic function. ... Cardiovascular function depends on the operational capacity of myocardial cells to generate the energy to expand and contract. Insufficient myocardial energy contributes significantly to CHF. Literally, heart failure is an "energy-starved heart." ... In simple terms, sick hearts leak out and lose vital ATP, and the endogenous restoration of ATP cannot keep pace with this insidious deficit and relentless depletion. When ATP levels drop, diastolic function -- the most important precursor of congestive heart failure -- deteriorates. D-ribose, L-carnitine, and CoQ10 act to promote cardiac energy metabolism and help normalize myocardial adenine nucleotide concentrations. These naturally occurring compounds exert a physiological benefit that directly affects heart function. As the rate-limiting compound, supplemental ribose supports ATP quantity in the synthesis of new ATP. CoQ10 and carnitine enhance the turnover of ATP in the inner mitochondrial chain. All are recommended as adjunctive metabolic therapies in the treatment of heart failure."
• June 2015: "Previous studies have demonstrated that diastolic function is energy dependent and supplemental D-ribose has shown to improve diastolic dysfunction. This study investigated what role D-ribose might play in congestive heart failure patients with preserved systolic function and diastolic dysfunction. ... Results: An improvement in their tissue Doppler velocity (E′), which was maintained at 9 weeks, was demonstrated in 64% of the patients."
• June 2016: "Myxomatous mitral valve disease (MMVD) is the most common cause of mitral valve regurgitation (MVR) in small animals, with a prevalence of approximately 30% in small breed dogs over the age of 10 years. The time course of the disease influences the clinical symptoms. If MVR occurs slowly, there is time for compensation and the animal may be asymptomatic; if MVR develops acutely, the clinical signs can be very severe. With MVR there is a decrease in stroke volume because part of the outflow of the left ventricle (LV) is directed back to the left atrium (LA). The regurgitant fraction causes volume overload of the LA and LV, according to Laplace's law. Volume overload increases wall stress, which in turn requires greater adenosine triphosphate (ATP) and O2 consumption. As a compensatory mechanism, myocardial hypertrophy occurs. The chronic volume overload of the LA can lead to dilation of the atrium, with increased pressure and pulmonary congestion, resulting in pulmonary oedema and congestive heart failure (CHF). Possible complications of MMVD are right-sided CHF, due to direct involvement of the tricuspid valve or as a consequence of pulmonary hypertension (PH), and arrhythmias, such as atrial fibrillation (AF), due to atrial enlargement."
• May 2017: "D-ribose is a carbohydrate (sugar) that is important for the body's cellular metabolism through the production of a substance known as ATP (adenosine trisphosphate). Patients suffering from severe heart disease, especially heart diseases such as dilated cardiomyopathy, may have severely depleted stores of ATP within heart muscle tissue. A small study in humans with congestive heart failure suggested that supplementation with ribose may improve heart muscle function."
• January 2018: " Mitochondria are important organelles referred to as cellular powerhouses for their unique properties of cellularenergy production. With many pathologic conditions and aging, mitochondrial function declines, and there is a reduction in the production of adenosine triphosphate. The energy carrying molecule generated by cellular respiration and by pentose phosphate pathway, an alternative pathway of glucose metabolism. D-ribose is a naturally occurring monosaccharide found in the cells and particularly in the mitochondria is essential in energy production. Without sufficient energy, cells cannot maintain integrity and function. Supplemental D-ribose has been shown to improve cellular processes when there is mitochondrial dysfunction. When individuals take supplemental D-ribose, it can bypass part of the pentose pathway to produce D-ribose-5-phosphate for the production of energy. In this article, we review how energy is produced by cellular respiration, the pentose pathway, and the use of supplemental D-ribose."
• March 2018: "Cardiovascular disease still remains the leading cause of deaths worldwide. ... All cells require adequate adenosine triphosphate (ATP) levels to maintain their integrity and function. ... D-ribose, a naturally occurring pentose carbohydrate, has been shown to increase cellular energy levels and improve function following ischemia in pre-clinical studies and have demonstrated potential benefits in clinical evaluations. This review paper presents an overview of ischemic cardiovascular disease and the potential role that D-ribose could play in improving myocardial energy levels and function in the area of ischemic cardiovascular diseases."
Our Blog: "D-ribose can boost the energy in MVD-affected hearts"
RETURN TO TOP
--- Omega-3 fatty acids
 Omega-3 polyunsaturated long-chain fatty acids
(omega-3s) have been found to have beneficial effects upon
humans and dogs in heart failure. They include EPA (eicosapentaenoic
acid) and DHA (docosahexaenoic acid), both of
which are found naturally in fish. In an
October 1998 study,
supplementation of fish oils containing EPA and DHA in dogs diagnosed
with heart failure decreased interleukin-1B (IL-1B)
concentrations and improved cachexia.
Omega-3 polyunsaturated long-chain fatty acids
(omega-3s) have been found to have beneficial effects upon
humans and dogs in heart failure. They include EPA (eicosapentaenoic
acid) and DHA (docosahexaenoic acid), both of
which are found naturally in fish. In an
October 1998 study,
supplementation of fish oils containing EPA and DHA in dogs diagnosed
with heart failure decreased interleukin-1B (IL-1B)
concentrations and improved cachexia.
In this December 2017 article, the investigators reported finding that the serum levels of interleukin 1B increases in MVD-affected dogs, and even higher levels are observed in dogs with MVD symptoms, and that there is a significant correlation between heart enlargement and congestive heart failure and the circulating levels of IL-1B.
Cachexia is discussed in some depth here. In short, it is the loss of muscle mass in MVD-affected dogs due to the inability of the heart to pump blood throughout the body. The most affected regions of the dog's body are the hind quarters, but cachexia can extend throughout the body, causing weight loss and severe weakness.
In this February 2025 article about case studies of two dogs diagnosed with ventricular aarrhythmias, omega-3 fattyacid supplementation in both cases resulted in adequate arrhythmia control when previous anti-arrhythmic medications had not.
While processed fish oils contain Omega-3s, a far more natural and un-processed source of Omega-3s is the sardine. An ounce or slightly less of sardines, added to four of the MVD-affected dog's meals per week, should provide sufficient Omega-3s in their natural and unprocessed state.
RETURN TO TOP
--- hawthorn
Hawthorn is an herb derived from the berry and other parts of the hawthorn shrub/tree. It is a cardiotonic which reportedly improves the general function of the heart, including regulating the heart beat and lowering blood pressure. See this September 2018 article. It can steady and strengthen a weak or irregular heartbeat. It can lower blood pressure and cholesterol. But it's most valuable attribute is its ability to support a dog with heart failure.
It has been found to be beneficial to dogs with MVD in several ways. It tends to dilate (widen) the blood vessels, allowing more blood to flow more readily, and doing so, it serves to increase the heart's output of blood and thereby improve circulation of the blood. Hawthorn is rich in flavinoid, the red pigment compound most responsible for its tonic effect on the smooth muscles. Hawthorn also is an anti-oxident, maintaining oxygen levels in the blood. It also is considered very safe for dogs to ingest with low toxicity. However, hawthorn performs in a manner similar to pimobendan, and so it should not be given in addition to pimobendan without the consent of the dog's cardiologist. Similarly, it should not be combined with digoxin.
Commercially prepared hawthorn comes as a liquid and/or powder. Animal Essentials offers a liquid formula called Heart Health Cardio Strength for Dogs and Cats.
RETURN TO TOP
--- magnesium
Mitral valve disease is associated with low levels of magnesium. Cavaliers as a breed have been found to have a high prevalence of hypomagnesemia, meaning low levels of magnesium in their blood ("plasma") and in their soft tissues and bone ("intracellular").
In a December 1998 article, veterinary cardiologists report finding hypomagnesemia in 30 cavalier King Charles spaniels which were diagnosed with MVD but not in heart failure. They found that 50% of the CKCSs had low levels of magnesium in their blood plasma, which they described as a "high prevalence". They apparently did not measure the magnesium levels in the dogs tissues, their "intracellular" magnesium.
The low magnesium status is not primarily due to a dietary deficiency since long-term oral magnesium supplementation does not fully correct the imbalance in cavaliers. However, magnesium supplementation has been partially successful.
RETURN TO TOP
--- salvia shou wu
Salvia Shou Wu is a proprietary combination of herbs (salvia root, polygonum, crataegus, peony (red), astragalus root, achyranthes, loranthus, tangkuei, dalbergia, licorice) by Seven Forests, which is designed to enhance blood circulation, dispense stagnant blood, and nourish the blood. Holistic veterinarians recommend this product for dogs in heart failure due to MVD, at the rate of 1/4 tablet twice daily with food, for dogs the size of cavaliers.
RETURN TO TOP
--- wu ling san
Wu Ling San is a proprietary blend of herbs (hoelen fungus, alisma root, polyporus, cinnamon, and atractylodes root) which is designed to promote urination and leaches out dampness. Holistic veterinarians recommend this product for dogs with enlarged hearts or in heart failure, due to MVD, at the rate of 1/4 teaspoon twice daily with food, for dogs the size of cavaliers.
RETURN TO TOP
-- supplements to avoid
- taurine
- L-carnitine
- choline
- medium chain triglycerides (MCTs)
- problems with compound heart supplements
There are some dietary supplements which are totally inappropriate for MVD-affected cavaliers because they have been found to worsen the progression of MVD. Unfortunately, these supplements are erroneously recommended by some veterinarians and particularly those who also are Internet vendors of canine supplements, as being beneficial to dogs. These potentially dangerous supplements include taurine, L-carnitine, and choline.
Other supplements that cavaliers in particular should avoid are medium chain triglycerides (MCTs), unless and until the dogs' DNA have tested and clear of MCAD (medium-chain acyl-coenzyme A dehydrogenase).
Finally, in this section, we discuss the hazards of relying upon commercial compound heart supplements which consist of a laundry list of insignificant quantities of ingredients which may or may not have any value in treating dogs with mitral valve disease, as opposed to some other heart disorder.
RETURN TO TOP
--- taurine
Bottom Line: Taurine is not an appropriate supplement for MVD-diagnosed dogs unless taurine concentrations in the dogs' blood plasma has been found to be too low.
If cavalier owners choose to ignore the advice to consult with an holistic veterinarian before giving their dogs supplements, they nevertheless should be aware of falsehoods about certain synthetic, chemical supplements, such as taurine. In its natural state, taurine is an amino-acid which dogs make themselves from other animo acids in their livers. All animals create taurine naturally in their own bodies, including mammals, fish, shellfish, and poultry, including eggs. It is found in their hearts, brains, retinae, and skeletal muscles. Natural taurine is found exclusively in animal proteins and is absent from plant-sourced proteins. If dogs consume fresh muscle meats from those sources in their diets, they will not need the artificial, chemical version of taurine sold as a supplement. Because commercial dry dog foods (kibble) destroy the building blocks of taurine in their meat ingredients during processing, synthetic taurine is added to those ingredients. Synthetic taurine is produced mostly in China, from the raw materials of ethylene oxide, sulfuric acid, and sodium bisulfite. See this May 2020 article.
Excessive dosages of synthetic taurine supplementation have been found to be toxic in rodent studies, causing extreme low and high blood pressure. Thus far, its safety in humans and canines has not been determined. So, giving taurine supplements to dogs amounts to experimenting on those dogs without any fore-knowledge of the risks involved. In general, over-supplementing -- by adding synthetic supplements to diets of dogs which do not need supplementation -- can impose an unnecessary additional burden upon the liver, kidneys, and the immune system.
Research studies have shown that MVD-affected dogs tend to have higher plasma taurine concentrations than unaffected dogs. In a 1995 study (by George A. Kramer, Mark D. Kittleson, Philip R. Fox, Julia Lewis, and Paul D. Pion), for example, "[P]lasma taurine concentrations were highest in dogs with AVD [acquired valvular disease, e.g, MVD] ... We conclude that plasma taurine concentrations may be increased in dogs with AVD."
In a 2002 presentation, Dr. Bruce Keene stated:
"Taurine supplementation is indicated whenever plasma or whole blood taurine concentrations are found to be low. ... [S]upplementation is generally only recommended after discovery of deficiency."
In a March 2022 article, USA veterinary nutritionists and cardiologists recommended that taurine supplementation be administered only to dogs with plasma or whole blood taurine concentrations which were low or borderline.
In a September 2022 article, Texas A&M veterinary school researchers examined the levels of taurine concentration in the blood of 200 cavalier King Charles spaniels in various stages of mitral valve disease (MVD). Twelve were in Stage A (meaning no MVD murmur); 150 were in Stage B1 (murmur but no enlargement); and 38 were in Stage B2 (murmur plus enlargement). None were in Stages C or D (heart failure). They report finding that taurine concentrations in both plasma and whole blood were "not significantly different", regardless of the stage of MVD and also regardless of the type of food being fed to the dogs. They devised reference intervals for whole blood taurine (152 to 373 μM) and plasma taurine (51 to 217 μM) concentrations in cavaliers.
In a July 2023 article, a team USA researchers tested 14 dogs, including 4 (29%) cavaliers, diagnosed with CHF due to MVD for a two-week period to see if taurine supplementation would suppress their renin-angiotensin-aldosterone system (RAAS). None of the dogs were taurine-deficient. They report finding that oral taurine supplementation did not have a suppressive effect upon the RAAS in this group of dogs with naturally occurring CHF secondary to MVD.
If the dog's blood plasma shows a deficiency of taurine, then supplementation at the rate of from 250 to 1000 mg twice daily may be necessary.
RETURN TO TOP
--- L-carnitine
Bottom Line: L-carnitine is not an appropriate supplement for MVD-diagnosed dogs unless L-carnitine concentrations in the dogs' blood has been found to be low.
Levocarnitine (L-carnitine) is a naturally produced amino-acid derivative and micronutrient. It is synthesized in the liver and brain and is released into the blood system. Most dogs make enough L-carnitine from the foods they consume. As with taurine, studies have shown that MVD-affected dogs, particularly those in heart failure (CHF -- Stage C or D) tend to have higher concentrations of L-carnitine in their blood serum, than do healthy dogs. It is not known if these higher concentrations mean that MVD-dogs do not need L-carnitine supplementation because they make more than enough on their own, or if MVD-dogs cannot synthesize it as sufficiently as can healthy dogs.
While several published studies have concluded that L-carnitine supplementation may be of value in treating dogs diagnosed with dilated cardiomyopathy (DCM) and humans with other heart disorders, to our knowledge there is to date no such veterinary literature finding that L-carnitine supplementation aids in slowing the progression of MVD in dogs, either before or after the onset of CHF. To the contrary, in a January 2019 article, MVD-affected dogs were found to have higher levels of L-carnitine in their bloodsteams than healthy dogs, and Stage C MVD-affected dogs had higher concentrationis of L-carnitine than did Stage B dogs.
The problem with L-carnitine and the reason to avoid supplementing it, is that it has been found to increase the amount of trimethylamine N-oxide (TMAO) in the dogs' blood. TMAO is an organic compound that is produced in the dog's gut from a combination of choline, carnitine, and betaine. MVD-affected dogs in heart failure have been shown to have significantly higher circulating levels of TMAO in their blood serum compared to healthy dogs and to dogs with pre-CHF MVD. See this January 2019 article and this April 2021 article.
In general, over-supplementing -- by adding synthetic supplements to diets of dogs which do not need supplementation -- can impose an unnecessary additional burden upon the liver, kidneys, and the immune system.
RETURN TO TOP
--- choline
Bottom Line: Choline is not an appropriate supplement for MVD-diagnosed dogs unless choline concentrations in the dogs' blood has been found to be low.
Choline is viewed as "an essential nutrient", implying it should be added to dogs' diets as a supplement. It is essential, but it is found naturally in most foods fed to dogs, including muscle meats, liver, eggs, and grains. Dry foods and some canned foods are laced with synthetic choline, choline chloride, as a supplement. So, dogs being fed meat-based and/or egg proteins and some grains are adequately nourished with all of the natural choline they need.
The problem with choline, and the reason to avoid supplementing it, is that it has been found (along with L-carnitine) to increase the amount of trimethylamine N-oxide (TMAO) in the dogs' blood. TMAO is an organic compound that is produced in the dog's gut from a combination of choline, carnitine, and betaine.
MVD-affected dogs in heart failure have been shown to have significantly higher circulating levels of TMAO in their blood serum compared to healthy dogs and to dogs with pre-CHF MVD. See this January 2019 article and this April 2021 article.
RETURN TO TOP
--- medium chain triglycerides (MCTs)
Bottom Line: Short of DNA testing for the MCAD-deficiency, the best solution for cavaliers would be to avoid dog foods which have MCTs as their primary oils (e.g., ketogenic diets, Purina Pro Plan Bright Mind, Purina Pro Plan CardioCare) and also MCT oil supplements (e.g., coconut oil).
Medium chain triglycerides (MCTs) are fats derived from coconut oil and palm kernel oil. They have been found to be more rapidly absorbed into the canine digestive system than other dietary oils.
Various claims have been made on the Internet websites of MCTs vendors, including so-called "natural solution" veterinarians, that MCTs made from "virgin coconut oil" will resolve dogs suffering from mitral valve disease. A veterinary journal article claims that a combination of supplements including MCTs was "able to slow or reverse cardiac changes in dogs with early, preclinical MMVD [mitral valve disease]." A dog food containing MCTs is marketed specifically for epileptic dogs, advertising that the MCTs will reduce seizure frequency. Another dog food is advertised that the MCTs in its ingredients "support cardiac function in dogs".
All that said, however, it appears that MCTs are a serious health hazard for a high percentage of cavalier King Charles spaniels. MCAD (medium-chain acyl-coenzyme A dehydrogenase) is an enzyme which is controlled by a specific gene in canines, called the ACADM gene. A mutation of that gene in dogs causes a MCAD-deficiency in the dogs with that mutation. MCAD-deficiency is a presumably inherited disorder called an organic aciduria that prevents the dog's body from converting certain fats to energy, particularly medium-chain fatty acids.
Two published studies suggest that MCAD-deficiency is fairly common among CKCSs. In a May 2007 article, veterinary neurologists report finding that a young cavalier, exhibiting seizures which were not controllable with anti-convulsants (potassium bromide, phenobarbital, and gabapentin), had an organic aciduria with excessively high urine excretion of hexanoylglycine. They diagnosed a MCAD-deficiency.
In an October 2022 study of 162 cavaliers, researchers found that 52 of them were carriers of the protein changing variant of ACADM, and another 12 CKCSs were homozygous mutant dogs - 39.5% of the 162 cavaliers in the study. That research was prompted by examination of a 3 year old, male neutered cavalier which displayed complex focal seizures and prolonged lethargy. The dog was found to have a single insertion deletion variant of ACADM causing the MCAD deficiency. The affected dog was treated with various dosages of levetiracetam and phenobarbital and was prescribed a low fat diet and a midnight snack consisting of carbohydrates. Prolonged periods of fasting and formulas that contained medium chain triglycerides as primary source of fat were also advised to avoid.
Fats are one of the three essential categories of food nutrients, the others being proteins and carbohydrates. In short, CKCSs affected with the MCAD-deficiency cannot digest MCTs, and so when MCTs are the primary or sole source of fats in their diets, they digest no fats. In humans, MCAD-deficiency has been found to lead to severe liver disorders, atrophy of the skeletal muscles, loss of consciousness, coma, and even sudden death.
The authors of the leading (November 2020) veterinary journal article finding that MCT oils improve dogs' cognitive abilities now advise that MCT-enriched diets may be inappropriate for some CKCSs, based upon the findings of the October 2022 study. They recommend having cavaliers tested for the ACADM mutation before feeding MCT-enriched diets for those dogs.
So, MCT oils, including coconut oil, should not be fed to any cavalier unless that dog first has been tested clear for the ACADM mutation causing the MCAD-deficiency. See our webpage, "Medium-chain acyl-coenzyme A dehydrogenase deficiency (MCADD) in the cavalier King Charles spaniel ", for more information.
RETURN TO TOP
--- problems with compound heart supplements
There are several compound heart supplements offered for dogs. They include such products as Thorne's Heart Health Formula (formerly Bio-Cardio) and Five Leaf Botanicals Canine Heart Health Program for Canine Heart Disease. These two and others are way too generalized and un-focused for treating cavaliers diagnosed with mitral valve disease (MVD).
They tend to have unnecessary ingredients, such as taurine and l-carnitine in sizeable quantities, and way too little quantities of CoQ10 and no d-Ribose. They are not focused specifically on MVD and therefore tend to include ingredients to deal with other cardiac issues, such as dilated cardiomyopathy (DCM) which is totally unrelated to MVD in cavaliers.
With the exception of one compound heart supplement, Standard Process Canine Cardiac Support, these compound supplements are not worth their expense in treating MVD-affected dogs.
RETURN TO TOP
-- stem cells
Stem cells are unspecified cells present through out the dog's body that are able to develop into numerous other cell types. Research in cardiac stem cell therapy is in early stages and is on-going. Researchers are dealing with issues such as the ever-pumping heart washing out stem cells which have been inserted into it.
In a 2008 pre-med research paper, the author suggests that injecting bone marrow stromal cells into the heart of a cavalier King Charles spaniel may stimulate stem cells to regenerate heart muscle and repair damage to the valve tissues. See also this February 2012 article.
In a 2014 report, veterinary researchers at North Carolina State University have successfully figured out a way to magnetize cardiac stem cells so that they are directed to the hearts of rats and remain there to perform therapeutic effects. The team has attached metalic nanoparticles from an FDA-approved drug, Feraheme, to cardiac stem cells and used a magnetic field to keep the cells in the heart. The process has resulted in a three-fold increase in cell retention in the rats' hearts.
Mesenchymal stem cells (MSCs) are particluarly useful in regenerative medicine, due to their existence in various organs of the dog's body and their ability to differentiate into may cell types for treatment of a variety of disorders, including spinal cord injuries, wound healing, and eye diseases.
Beginning in 2016, a research team at Tufts' University's Cummings
veterinary school, led by Dr. Andrew
 Hoffman
and veterinary cardiologist Dr. Vicky Yang (right), has been conducting a study of
ten MVD-affected dogs in congestive heart failure (determined by fluid
in the lungs), which are being injected with mesenchymal stem
cell (MSC) treatments to determine, first, if the therapy is
safe for the dogs, and, second, will result in improved cardiac
function, as determined by echocardiography, cardiac biomarkers, and
quality of life. The study is titled, "Allogeneic Umbilical Cord
Tissue (Wharton Jelly) Derived Mesenchymal Stem Cell Therapy for Chronic
Valvular Disease and Associated Congestive Heart Failure".
Hoffman
and veterinary cardiologist Dr. Vicky Yang (right), has been conducting a study of
ten MVD-affected dogs in congestive heart failure (determined by fluid
in the lungs), which are being injected with mesenchymal stem
cell (MSC) treatments to determine, first, if the therapy is
safe for the dogs, and, second, will result in improved cardiac
function, as determined by echocardiography, cardiac biomarkers, and
quality of life. The study is titled, "Allogeneic Umbilical Cord
Tissue (Wharton Jelly) Derived Mesenchymal Stem Cell Therapy for Chronic
Valvular Disease and Associated Congestive Heart Failure".
In a December 2016 article, Thai researchers harvested dental pulp stell cells from the baby teeth of dogs and then injected the cells intravenously into 10 dogs diagnosed with heart failure due to mitral valve disease (none being CKCSs). Following 60 days of periodic examinations, they reported finding that the 10 dogs had measurable improvement in left ventricular ejection fraction and significantly improved quality of life, while in a 10 dog control group, none showed any such improvements. They concluded that "This finding suggests that pDSCs could be used as an alternative treatment for valvular heart disease in dogs."
In an October 2024 article, Korean researchers tested 6 dogs diagnosed with Stage B1 of MVD -- none being cavaliers -- with mesenchymal stem cells (MSCs) derived from ovaries (gonadal) tissues obtained from donor female dogs. The aim of the study was to assess the effectiveness of mesenchymal stem cell therapy in canines diagnosed with early stage mitral valve disease. The MSCs were intravenously administered monthly for 5 or more sessions. The 6 dogs were compared to 10 other dogs diagnosed with Stage B1 MVD, the control group, at the 1-year point and each year thereafter for 4 years. They concluded:
"In conclusion, therapy with MSCs derived from gonadal tissue significantly delayed MMVD progression by maintaining the early-stage for a longer period. Therefore, MSC therapy is considered a safe and effective treatment for patients with MMVD stage B1 who are managed without treatment."
 Dr.
Philip Richard Vulliet (left), veterinary medical director at
ReGena-Vet Laboratories and professor at UC Davis, has investigated the
potential of injecting adult bone marrow stem cells in MVD-affected dogs,
including cavaliers,
with the goal of slowing the progression of MVD and even shrinking the
size of the enlarged hearts. The lab currently is experimenting with
Doberman Pinschers affected with dilated cardiomyopathy. His contact
information is: ReGena-Vet Laboratories, LLC, 2079 Anderson Rd., Suite
B, Davis, CA, 95616, Tel: 530-902-9006, Fax: 530-756-0459, email
info@regenavetlabs.com or regenavetlabs@gmail.com, website regenavet.com
Dr.
Philip Richard Vulliet (left), veterinary medical director at
ReGena-Vet Laboratories and professor at UC Davis, has investigated the
potential of injecting adult bone marrow stem cells in MVD-affected dogs,
including cavaliers,
with the goal of slowing the progression of MVD and even shrinking the
size of the enlarged hearts. The lab currently is experimenting with
Doberman Pinschers affected with dilated cardiomyopathy. His contact
information is: ReGena-Vet Laboratories, LLC, 2079 Anderson Rd., Suite
B, Davis, CA, 95616, Tel: 530-902-9006, Fax: 530-756-0459, email
info@regenavetlabs.com or regenavetlabs@gmail.com, website regenavet.com
Nevertheless, finding an effective and safe delivery method for stem cell therapies remains difficult. Non-selective injection into veins is not viable, due to a lack of targeted delivery to the heart. Injection directly into the heart risks obstructing blood vessels. In a March 2004 article, the researchers found that injecting MSCs into a coronary artery of dogs caused acute myocardial ischemia (stroke). A possibly successful method may be catheterization of a cardiac vein, resulting in the injected cells being successfully retained in the heart. See this March 2025 article.
RETURN TO TOP
-- growth differentiation factor 11 (GDF11)
A protein called "growth differentiation factor 11" (GDF11) has been found to have a restorative effect in the hearts of aged mice. In a May 2013 study, the protein was injected into the blood systems of mice with hearts enlarged due to cardiac hypertrophy, for 30 days. At the end of the treatment. their hearts were significantly smaller than those in the control group. The researchers found that the treated mice' heart cells had shrank significantly.
RETURN TO TOP
-- new DNA injections
Researchers at the Wyss Institute and Harvard Medical School announced in July 2017 that they are developing a novel cardio-protective gene therapy to stop the progression of heart failure in dogs. As part of the technical development, the Wyss Institute is planning to launch a study in MVD-affected dogs. According to the developers, new DNA segments are to be injected into the MVD-affected dogs with cardiac enlargement (Stage C), which lead to the production of a "beneficial" protein intended to suppress fibrotic processes and halt the build-up of scar tissue in the heart. See this linked article for more information.
RETURN TO TOP
-- inflammation
MVD appears to be associated with a chronic state of inflammation, as evidenced by measurements of immunoglobulin antibodies and glycoprotein and complement proteins particularly associated with immune responses to inflammation. Therefore, among the treatments to consider would be methods of reducing inflammation by diets and supplements.
RETURN TO TOP
-- physical exercise
 There is very little published research regarding the advisability of
physically exercising MVD-affected dogs, by such things as walks,
treadmill running (right), or training for events such as agility. In an
October 2018 article, a team of Thai veterinary researchers
tested six Beagles with Stage B1 mitral valve disease (mitral
regurgitation but no heart enlargement), to determine the effects of
"submaximal endurance training" by treadmill running and 6-minute walks,
over a period of eight weeks. They report finding that the exercise:
There is very little published research regarding the advisability of
physically exercising MVD-affected dogs, by such things as walks,
treadmill running (right), or training for events such as agility. In an
October 2018 article, a team of Thai veterinary researchers
tested six Beagles with Stage B1 mitral valve disease (mitral
regurgitation but no heart enlargement), to determine the effects of
"submaximal endurance training" by treadmill running and 6-minute walks,
over a period of eight weeks. They report finding that the exercise:
• significantly improved the physical capacity of the dogs,
• did not affect cardiac function, and
• significantly reduced serum creatine.
They concluded that, "supervised or moderate regular exercise that is within the individual physical capacity should be performed in subclinical MMVD dogs and dogs with low routine physical activity, in order to preserve or improve physical capacity and quality of life." This study was limited to fairly sedentary MVD-affected dogs in Stage B1. It did not test dogs in Stage B2 or in Stage C.
RETURN TO TOP
Medications
 Stage B1 (murmur & no enlargement)
Stage B1 (murmur & no enlargement)- Stage B2 (enlarged left side of heart)
- Stage C (heart failure)
- Stage D (end stage of MVD)
- Experimental drugs
The major means of treating mitral valve disease in dogs is called "maintenance" and consists of medicating the dog with drugs. There is no pharmaceutical medication that can cure MVD. The most that can be hoped for in using any of these prescription drugs is to attempt to delay the progression of the consequences of the damage that MVD does to the mitral valves and the rest of the heart and the lungs. This is called "palliative care" and "suppressive care".
The severity of the dog's MVD determines whether any drugs, and which of them, should be administered and in what quanitities. Since the ACVIM's 2009 Consensus Statement was published, the severity of MVD has been divided into "stages" -- B1, B2, C, D -- what follows are discussions of which medications typically are prescribed for each stage of MVD.
-- Stage B1 -- murmur & no enlargement
 A cavalier with early mitral valve disease has a mild murmur
(usually at Grade 1 or 2 out of 6) but
the heart is not enlarged and the dog is symptom-free (asymptomatic). This dog would be at Stage B1 of
the ACVIM's 2009
Consensus Statement,
although a dog with even a higher grade murmur (Grade 3 or 4) could meet the
Stage B1 definition, as long as it is symptom-less and has no heart enlargement. Cardiologists refer to this as the
pre-clinical stage. At this stage, there is no need for treatment,
but heart size should be monitored by x-rays every 6 to 12 months.
Overweight dogs should be put on a weight-reducing diet. Low salt diets have
been suggested, to help reduce water retention. It would be prudent to avoid
extreme exertion.
A cavalier with early mitral valve disease has a mild murmur
(usually at Grade 1 or 2 out of 6) but
the heart is not enlarged and the dog is symptom-free (asymptomatic). This dog would be at Stage B1 of
the ACVIM's 2009
Consensus Statement,
although a dog with even a higher grade murmur (Grade 3 or 4) could meet the
Stage B1 definition, as long as it is symptom-less and has no heart enlargement. Cardiologists refer to this as the
pre-clinical stage. At this stage, there is no need for treatment,
but heart size should be monitored by x-rays every 6 to 12 months.
Overweight dogs should be put on a weight-reducing diet. Low salt diets have
been suggested, to help reduce water retention. It would be prudent to avoid
extreme exertion.
The participating cardiologists in the ACVIM's 2009 Consensus Statement unanimously declined to recommend any drug or dietary therapy for Stage B1 dogs. Nevertheless, studies of drugs have been conducted on dogs in Stage B1, and what follows is a discussion of the reports of those studies:
In a 2013 presentation titled "Medical Therapy of Congestive Heart Failure: The Essentials", Dr. Matthew W. Miller, board certified veterinary cardiologist, concisely summarized the current treatment protocol for cavaliers not yet in heart failure (Stage C):
"Treatment of the asymptomatic dog with a murmur caused by endocardiosis is not currently recommended unless there is evidence of impending heart failure (dramatic cardiomegaly and pulmonary venous distension). Scandinavian studies in the CKCS dog have failed to reveal any benefit in asymptomatic dogs; results from a North American study suggest a possible benefit, but were by no means conclusive."
RETURN TO TOP
--- supplements
Vitamins and food supplements such as these listed above may be prescribed for all stages of mitral valve disease. Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
RETURN TO TOP
--- ACE-inhibitors in Stage B1
 Angiotensin
converting enzyme inhibitors (ACE-inhibitors or ACE-I) in humans
have been found to widen blood vessels by relaxing the smooth muscle cells
in the vessels' walls (vasodilation), counteract fluid retention, and blunt
heart enlargement due to MVD. However, in dogs with Stage B1 MVD (or Stage
B2), and particularly in cavaliers, the
results have been more mixed and much less favorable. In fact, the most
recent studies have concluded that
ACE-inhibitors are worthless, even in cases of heart failure (Stage C).
Angiotensin
converting enzyme inhibitors (ACE-inhibitors or ACE-I) in humans
have been found to widen blood vessels by relaxing the smooth muscle cells
in the vessels' walls (vasodilation), counteract fluid retention, and blunt
heart enlargement due to MVD. However, in dogs with Stage B1 MVD (or Stage
B2), and particularly in cavaliers, the
results have been more mixed and much less favorable. In fact, the most
recent studies have concluded that
ACE-inhibitors are worthless, even in cases of heart failure (Stage C).
Since the ACVIM Consensus Statement does not recommend any drugs for Stage B1 dogs, there should be no reason to prescribe an ACE-inhibitor to a cavalier in Stage B1 of MVD.
RETURN TO TOP
--- alpha & beta blockers in Stage B1
As noted, the ACVIM Consensus Statement does not recommend any drugs for Stage B1 dogs. Nevertheless, other drugs being used by some veterinary cardiologists are carvedilol (Coreg), and bisoprolol, both non-selective beta-and alpha-blockers with anti-oxidant effects -- also known as beta- (B-) adrenergic receptor antagonists (BARA) -- which reduce the heart's rate and the force of its contraction, thereby reducing the work of the heart. Carvedilol and bisoprolol also cause the arteries to relax and the blood pressure to drop. A few cardiologists have begun to administer low doses of carvedilol and Bisoprolol early in the disease process, in hopes of causing MVD to progress at a slower rate than dogs not taking the medication. In a 2009 study, it has been suggested that BARA have the potential to slow the progression of the mitral valve's degeneration by interfering with the serotonin signaling pathway -- a possible major factor in MVD progression -- and by reducing the "wear and tear" of the valve by reducing the pressure differences between the left ventricle and atrium.
To learn more about alpha and beta blockers, go to our discussion of them in our Treatment - Stage B2 section.
RETURN TO TOP
--- pimobendan in Stage B1
As noted above, the members of the ACVIM panel who participated in its 2009 Consensus Statement unanimously refused to recommend prescribing pimobendan to Stage B1 dogs.
Pimobendan (Vetmedin, Cardisure, Safeheart, Pimocard, Pimomedin, Pimotab, Zelys), has been prescribed for MVD-affected dogs in heart failure (Stage C) and, in some cases, dogs with left-side heart enlargement (Stage B2). It should not be prescribed for cavaliers in Stage B1. In a September 2016 article, an international team of veterinary cardiologists published the EPIC Study report, which tested the dosing of pimobendan on MVD-affected dogs in Stage B2 (discussed below). They do not recommend prescribing pimobendan for dogs in Stage B1. Also, harmful side effects have been noted from early use of this drug. See the "A Few Words About Pimobendan" box below for details. The US Food & Drug Administration (FDA) has approved the administration of pimobendan only for MVD-affected dogs in heart failure (Stage C) and not for dogs in either Stage B1 or B2.
To learn more about pimobendan, go to our discussion of it in our Treatment - Stage B2 section.
RETURN TO TOP
--- sildenafil (Viagra, Revatio) in Stage B1
Sildenafil (Viagra, Revatio) (a/k/a sildenifil) is a selective phosphodiesterase-5 inhibitor that has been demonstrated to delay ventricular enlargement in humans and experimental animals. In MVD-affected dogs, this drug has been prescribed for MVD-affected dogs already in heart failure (Stage C) if pulmonary hypertension is also detected. Pulmonary arterial hypertension, as a complication of mitral valve disease, is higher diastolic or systolic pulmonary arterial pressure than normal pressure. This high pressure may lead to increased right ventricular pressure and right atrial chamber enlargement, leading to possible right side heart failure.
In a March 2017 report of a 180-day study of 30 MVD-affected dogs (none were cavalier King Charles spaniels) in Stage B1 or Stage B2 (non-symptomatic), a team of Thailand investigators treated half of the group with sildenafil (Viagra, Revatio) (a/k/a sildenifil) versus a control group. They found:
• The stroke volume (the volume of blood pumped from the left ventricle per beat) at day 30 was significantly higher in the sildenafil group
• The LA/Ao (left atrial-to-aortic ratio) and the MR jet area were significantly lower beginning at day 30, day 90, and day 180.
• The 2D-LA (2-dimentional left atrium size) was significantly lower at day 90 when compared with control group.
• The differences of NTproBNP (a natriuretic peptides test biomarker in cardiac disease) from baseline were significantly lower when compared with control group at the same timepoint, day 90 and day 180. (Click here for details about natriuretic peptides tests.)
They concluded:
"In conclusion, this study suggested that long-term treatment with sildenafil prevented aggravation of disease progression as suggested by several echocardiographic indices (i.e. SV, LA/Ao, MR jet area, 2D-LA) and reduced NTproBNP level at the indicated timepoints in dogs with asymptomatic mitral valve degeneration."
To learn more about sildenafil, go our discussion of it in our Treatment - Stage B2 section.
RETURN TO TOP
-- Stage B2 -- enlarged heart
- Pimobendan
- Amlodipine
- Sildenafil (Viagra, Revatio)
- Diuretics
- ACE-inhibitors
- Spironolactone
- Angiotensin receptor blockers (ARBs)
- Alpha & beta blockers
- D-Ribose in Stage B2
- Other drugs for treating Stage B2
- Bronchial dilators for coughing
- Avoid vaccines
 The
next stage in the progression of MVD is indicated (usually) by a louder murmur and
by
enlargement of the heart on x-rays or echo scan. At this stage, reducing exercise will help to reduce the heart's
workload. This is a Stage B2 dog, according to the 2009 ACVIM
Consensus Statement.
Stage B2 encompasses all variations of enlargement -- mild, moderate, and
severe -- and enlargement due to either or both the heart's left atrium and
the left ventricle.
The
next stage in the progression of MVD is indicated (usually) by a louder murmur and
by
enlargement of the heart on x-rays or echo scan. At this stage, reducing exercise will help to reduce the heart's
workload. This is a Stage B2 dog, according to the 2009 ACVIM
Consensus Statement.
Stage B2 encompasses all variations of enlargement -- mild, moderate, and
severe -- and enlargement due to either or both the heart's left atrium and
the left ventricle.
A majority of the participating cardiologists in the ACVIM's 2009 Consensus Statement declined to recommend any drug or dietary therapy for Stage B2 dogs, apart from an ACE-inhibitor. Nevertheless, some cardiologists begin prescribing other medications at this stage, and studies of drugs have been conducted on dogs in Stage B2. What follows is a discussion of the reports of those studies.
--- pimobendan in Stage B2
BOTTOM LINE: It may be appropriate to start pimobendan if the heart's left atrium and/or left ventricle is moderately to severely enlarged, or if either of them is only mildly enlarged but the percentage of mitral regurgitation is over 50%.
The recommended starting dosage of pimobendan is 0.25-0.3 mg per kg of body weight (1 kg = 2.2 lbs).
Pimobendan typically is prescribed every 12 (or 8) hours on an empty stomach. The Vetmedin website recommends giving it an hour before meals. A recent meal reportedly significantly decreases absorption.
Since 2009, the only drug which has been found by peer-reviewed
veterinary studies to be effective in treating
 MVD-affected dogs in
Stage B2 is pimobendan
(Vetmedin, Cardisure, Safeheart, Pimocard, Pimomedin, Pimotab, Zelys). In
2022, the US Food & Drug
Administration (FDA) "conditionally" approved the administration of pimobendan
for MVD-affected dogs in
Stage B2. The makerof Vetmedin is conducting
a study called
EPOCHAL to meet the FDA's requirements for removing the
"conditional" aspect of its approval.
MVD-affected dogs in
Stage B2 is pimobendan
(Vetmedin, Cardisure, Safeheart, Pimocard, Pimomedin, Pimotab, Zelys). In
2022, the US Food & Drug
Administration (FDA) "conditionally" approved the administration of pimobendan
for MVD-affected dogs in
Stage B2. The makerof Vetmedin is conducting
a study called
EPOCHAL to meet the FDA's requirements for removing the
"conditional" aspect of its approval.
Pimobendan works in two separate ways. First, it relaxes and widens the blood vessels, allowing more more blood to flow from the heart in the proper direction, thereby reducing the congestion of blood in the heart and reducing the blood pressure. This is called vasodilating. Second, it strengthens the heart muscle, enabling it to pump more efficiently. This is called an inotropic effect, and it works on the heart muscle's contractility.
Technically speaking, pimobendan is a benzimidazole pyridazinone derivative and is classified as an inodilator (a calcium sensitizer and phosphodiesterase-III inhibitor and a positive inotrope) and arteriovenous dilator, which reportedly has a potent, positive affect upon the force with which the heart muscle contracts, and also mildly eases the resistance in the circulatory system by dilating blood vessels. When pimobendan metabolizes in the patient's stomach, its metabolite is O-desmethyl-pimobendan (ODMP).
Pimobendan typically is prescribed at the dosage rate of 0.2 to 0.3 mg/kg (1 kg = 2,2 lbs) every 12 hours on an empty stomach. The Vetmedin website and its Client Information Sheet (below) recommend giving it an hour before meals. A recent meal reportedly significantly decreases absorption.

Pimobendan may have any of several disturbing side effects. A list of its adverse reactions, published bythe maker of its brand name, Vetmedin, is here:
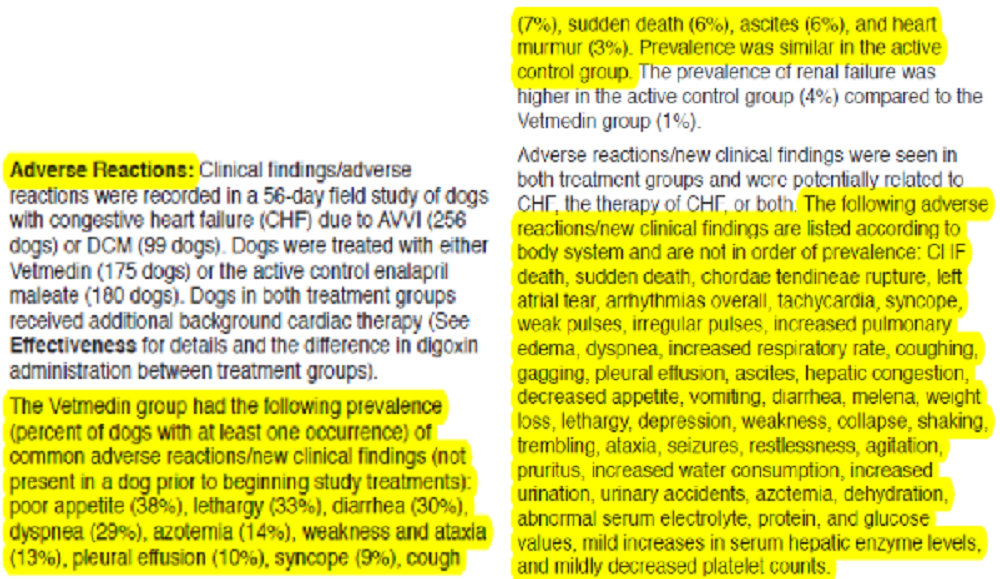
Depending upon the serverity of the reaction, the cardiologist may recommend reducing the dosage or eliminating the drug.
The 2016 EPIC Study report:
In a November 2016 article (the EPIC Study), an international team of veterinary cardiologists report that the administration of pimobendan to MVD-affected dogs with echocardiographic and radiographic evidence of specific minimum degrees of heart enlargement results in prolonging the pre-heart failure period by approximately 15 months over non-treatment (a placebo), which represents a substantial clinical benefit. (Note that this study was entirely funded by the manufacturer of pimobendan.)
To be included in the study, a dog had to be 6 years of age or older, have a mitral valve murmur of at least Grade 3 of 6, have echocardiographic evidence of advanced MVD consisting of characteristic valvular lesions of the mitral valve, regurgitation through the mitral valve (MR) on the color Doppler echocardiogram, and have specific minimum degrees of echocardiographic evidence of left atrial (LA/Ao] > 1.6) and left ventricular (LVIDDN > 1.7) dilation, plus radiographic evidence of a vertebral heart size (VHS) > 10.5. The researchers warn that:
"... it should be borne in mind when interpreting these results that all dogs included in the analyses also met the echocardiographic inclusion criteria and these results therefore might not be generalizable to all dogs with a VHS > 10.5 in the absence of concurrent echocardiographic measurements and a confirmed diagnosis of MMVD."
Therefore, to apply the results of this study to future treatment of MVD-affected dogs, an x-ray of the heart, showing a VHS over 10.5, is not sufficient by itself, and that the two echo measurements must also be confirmed. In addition, none of the participating dogs were being treated with any other cardiac medication. This raises the question as to the advisability of prescribing ACE-inhibitors prior to or while treating Stage B2 dogs with pimobendan.
Of 354 dogs in the EPIC Study, 161 (45.5%) were cavaliers. Of those 354 dogs, 178 of the dogs were treated with pimobendan (approximately 81 CKCSs), and 180 received the placebo (about 80 cavaliers). Of those 178 dogs treated with pimobendan, 59 (33.1%) reached congestive heart failure (CHF), 15 (8.4%) died of cardiac-related deaths during the treatment. The authors explained that:
"Although a greater number of dogs in the pimobendan group experienced spontaneous cardiac death (12 versus 5), the proportion of dogs in each group experiencing this event was not significantly different."
They concluded:
"Chronic oral administration of pimobendan to dogs with echocardiographic and radiographic evidence of cardiomegaly secondary to MMVD, in the absence of concurrent cardiovascular medication, results in the prolongation of the preclinical period, and is safe and well tolerated. The median time to the onset of CHF or cardiac-related death was prolonged by approximately 15 months, and the risk of a dog experiencing this event was reduced by approximately one-third; the majority of the benefit observed was attributable to delaying the onset of CHF. This substantial degree of prolongation of the preclinical period is f clinical relevance and is of importance to veterinarians and owners of dogs affected by this common disease."
So, the bottom line for starting pimobendan in Stage B2, according to this EPIC Study, is:
(1) The dog must be in Stage B2 of mitral valve disease; AND
(2) Have a murmur of at least Grade 3; AND
(3) The dog must NOT be on any other cardiac medication; AND
(4) An echocardiogram must be conducted and show valvular lesions of the mitral valve, regurgitation through the mitral valve (MR) on the color Doppler echocardiogram, and have echocardiographic evidence of left atrial and left ventricular dilation (defined as a left atrial-to-aortic root ratio [LA/Ao] equal to or greater than 1.6 and body weight normalized left ventricular internal diameter in diastole [LVIDDN] equal to or greater than 1.7); AND
(5) X-rays showing evidence of enlargement with a vertebral heart size (VHS) greater than 10.5.
This essentially means that once the MVD-affected dog develops a Grade 3 murmur and an x-ray showing enlargement, the owner should have a board certified veterinary cardiologist perform the echo exam. However, published research, confirmed by admissions made by two of the EPIC Study's lead investigators, has demonstrated that the EPIC Study's minimum measurements of enlargement really are within the range of normal sized hearts for cavaliers, meaning those in Stage B1. WARNING: Therefore, the EPIC Study's parameters should be ignored, and CKCSs' cardiologists should determine whether or not the Stage B dog really does have enlargement, rather than just a large normal size heart. This can be done by comparing baseline x-rays with current ones, or baseline echocardiogram measurements with current ones and/or comparing the shape of the cavalier's heart with the known shape of a normal heart, on x-rays.
Additionally, the EPIC Study's article is sloppily worded, leading to frequent confusion among prescribing veterinarians as to when to intiate the medication. The article may be (and, indeed, has been) interpreted in two very conflicting manners. The first, and we think the intended,manner of its authors, is to commence pimobendan when the dog's left chambers are at least moderately to severely enlarged, and not just mildly or minimally larger than normal. However, because the EPIC Study article is so loosely worded, it has been interpreted by some vets to mean that if the heart is the least bit enlarged, that pimobendan treatment should be commenced.
Critical analysis of the EPIC Study:
Notwithstanding the recommendations of the EPIC Study, several cardiologists warn against starting pimobendan if the amount of enlargement of the left atrium is only mild, meaning that the degree of mitral regurgitation (MR) likewise is mild. They recommend waiting until the amount of MR is at least moderate to severe before prescribing pimobendan to dogs in Stage B2. Their reasoning is two-fold. First, the data from the EPIC Study indicates that study's dogs with only mild MR did not benefit any more from the administration of pimobendan than did the dogs with greater degrees of MR. Second, these cardiologists point out that the condition of many dogs with only mild MR will never reach heart failure (Stage C), and therefore there would be no point in starting those dogs on pimobendan to begin with. Pimobendan is an expensive drug, and it would be financially unfair to expect owners of dogs which may never reach Stage C to start dosing that pill twice daily for the rest of those dogs' lives.
Another advantage of waiting to see if the degree of enlargement of the heart reaches moderate to severe is that more than one echocardiogram would be performed before confirming the extent of the enlargement. A disadvantage of a single echocardiogram is that, while enables the operator to measure the dimensions of the left atrium and left ventricle, it does not, by itself, determine whether either of those chambers are enlarged. If two or more echos are performed over a period of several months, then the operator can confirm by comparing measurements whether the heart indeed has enlarged, as well as how rapidly that enlargement is taking place.
2019 ACVIM Consensus Statement:
A majority of the ACVIM panel who participated in its 2009 Consensus Statement did not recommend prescribing pimobendan to Stage B2 dogs, but that was prior to the 2016 EPIC Study. The U.S. Food and Drug Administration's (FDA) 2007 report approving the use of pimobendan for dogs also contains the warning that the drug not be prescribed by dogs which are not in heart failure. On each container of Vetmedin is the warning that "Vetmedin should not be given in cases ... where an augmentation of cardiac output is inappropriate for functional or anatomical reasons. Warnings: Only for use in dogs with clinical evidence of heart failure. However, research reports are conflicting, and harmful side effects have been noted from early use of this drug. See the "A Few Words About Pimobendan" box below for details.
The 2009 Consensus Statement was revised in an April 2019 ACVIM Consensus Statement, to take into account the results of the 2016 EPIC Study. The main changes have been to re-define Stage B1 and Stage B2. In short, the new Stage B1 is determined by whether echocardiographic measurements of either the dog's left atrium or left ventricle is less than the EPIC Study's species-wide cut-off dimension. If one of those measurements, say of the left atrium (LA), is equal to or above that cut-off measurement (which is LA/Ao = 1.6), but the other measurement, of the left ventricle (LV), is less than the species-wide LV cut-off measurement (LVIDDN = 1.7), then by this 2019 definition of Stage B1, the dog's heart is not deemed to be enlarged, and therefore administering pimobendan would not be appropriate. The 2019 ACVIM definition of Stage B2 is the corollary of the new Stage B1. In other words, an MVD-affected dog's heart is not deemed to be enlarged unless both its LA and its LV dimensions are equal to or greater than those two EPIC Study species-wide cut-off dimensions. This revision of Stages B1 and B2 and reliance upon both the LA and LV dimensions exceeding the EPIC Study's arbitrary mininum measurements could also result is a dog with both an enlarged LA and LV not being placed in Stage B2 because, even though its chambers are enlarged, neither of them are large enough to meet the species-wide minimum measurements of the new definitions. (See this chart.)

Several research articles have found that the 2019 ACVIM Consensus Statement's definitions of enlarged LAs and LVs are inaccurate for certain breeds of dogs, particularly for cavaliers and small toy breeds, such as the toy poodle and chihuahua. The danger pointed out in these articles is that if the 2019 ACVIM Consensus Statement's definition is followed, dogs of some breeds with severely enlarged hearts may be denied pimobendan medication while dogs of some other breeds may be started on pimobendan when their hearts are of normal size. Ideally, if the treating cardiologist has been able to compare earlier measurements of the dog's heart with current ones -- either from x-rays or echo scans -- then an accurate determination can be made of whether the dog's LA and/or LV actually has enlarged in the interim.
Interestingly, neither in the EPIC Study nor anywhere else have researchers been able to determine why pimobendan appears to prolong the time before MVD-affected dogs reach heart failure. Some have speculated that the drug improves blood flow through the heart -- its vasodilating effects. Others surmise that it keeps the mitral valve leaflets tighter and thereby reduces mitral regurgitation.
RETURN TO TOP
--- amlodipine in Stage B2
Amlodipine besylate (Amodip, Norvasc) is a long acting vasodilator, meaning that it can decrease blood pressure by relaxing smooth muscles and widening blood vessels, allowing more blood to flow through them. Unlike pimobendan, it does not also have inotropic effects but as a vasodilator, it is much more potent than pimobendan and also considerably less expensive. Amlodipine acts by calcium channel blockage. It decreases resistance to the forward flow of blood, which results in a decrease in the back flow of blood from the left ventricle through the mitral valve into the left atrium (mitral regurgitation), and thereby decreases pressure in the left atrium. High doses of amlodipine are necessary for it to be effective, up to 1 mg/kg/day. However, initial dosages normally start at around 9.1 mg/kg/orally per day. Blood pressure checks before starting this drug, and over the course of administrating it (as doses may need to be increased), are necessary.
Concurrent ACE-inhibitor medication is advised, as there is some evidence that at high dosages, amlodopine may activate the RAAS in healthy dogs.
In a June 2003 study of sixteen dogs in Stage C with moderate to severe mitral regurgitation (MR), treatment with amlodipine reduced systolic blood pressure (9.6% decrease) and severity of MR (15.1% decrease in regurgitant fraction) and the dimension of the left ventricle (3.5% decrease in LV end-diastolic dimension). However, no significant change of the echographic LA/Ao-ratio was detectable in that study. The investigators concluded that the drug can be used simultaneously with standard cardiac therapy, which would include ACE-inhibitor, Lasix, spironolactone, and digoxin in Stage C. Amlodipine reportedly may cause gingival hyperplasia with chronic administration in dogs, with a reported incidence of 8.5%.
See, also, this 2012 comparative study of amlodipine versus benazepril. In a 2011 study, the Canadian researchers concluded that:
"Combining furosemide, ACEI, pimobendan, spironolactone, and amlodipine may result in long survival times in dogs with MR [mitral regurgitation] and CHF."
However, combined use of CCBs and ACE-inhibitors is controversial because of concerns for systemic hypotension and worsening renal function. Also, overdoses of CCBs can cause serious adverse effects on the cardiovascular system. See this September 2015 report specifically on amlodipine.
In a May 2017 report of a study involving 21 MVD-affected dogs in Stage C, a team of Brazilian researchers compared the treatment of CHF dogs with either pimobendan or amlodipine. They found no statistically significant differences in measurements of systolic blood pressure, or electrocardiographic parameters, or diastolic function using echocardiographic parameters. They concluded that neither pimobendan nor amlodipine appears to be superior to the other. Because of those results, the invesigators speculated that the clinical benefits of pimobendan in Stage C of MVD may be due to its vasodilating properties rather than its inotropic effects.
In a February 2022 article, South Korean researchers found in one Yorkshire terrier with multiple disorders (hypertension, pancreatitis, Cushing's disease, Stage B1 of MVD, chronic kidney disease, and a previous cerebellar infarction (stroke), that a high dose of amlodipine (0.4 mg/kg PO q12h) was the cause of pleural effusion, which is the build-up of excess fluid between the layers of the pleura membranes outside of the lungs. The pleura line the lungs and the inside of the chest cavity, and normally, there is only a small amount of fluid in the pleura. When the dosage of amlodipine was reduced to 0.3 mg/kg PO q12h, the symptoms of pleural effusion disappeared within 24 hours, and by the fifth day, no pleural effusion was identified on x-rays.
In an April 2022 article, South Korean veterinary cardiology researchers added the calcium-channel blocker amlodipine to the current medications of 24 small breed dogs (none being cavalier King Charles spaniels) all diagnosed with Stage B2 or C of mitral valve disease (MVD), for a period of 7 days. They found no adverse effects, and that several measurements of MVD, including left ventricular end-diastolic internal diameter (LVIDD), left ventricular end-diastolic diameter normalized for bodyweight (LVIDdN), left atrial diameter (LAD), and E wave reduced statistically after the week of amlodipine treatment. The LVIDdN were decreased by 8% (2.2 mm); the LA/Ao ratio, was decreased by 6.4% (1.57 mm); the E wave was decreased by 14% (0.18 m/s). They concluded that: "These findings suggest that low-dose amlodipine should be considered as treatment for dogs with ACVIM stage B2-C MMVD."
Side effects may include gastro-intestinal disorders and excessively low blood pressure (hypotension). Amlodipine also has been found to cause gingival hyperplasia (the abnormal growth of excessive gum tissue) in some dogs. See this January 2009 article. See this September 2015 Plumb's overview for more details.
RETURN TO TOP
--- sildenafil (Viagra, Revatio) in Stage B2
Sildenafil (Viagra, Revatio) (a/k/a sildenifil) is a selective phosphodiesterase-5 inhibitor (PDE5i) that has been demonstrated to delay ventricular enlargement in humans and experimental animals. In MVD-affected dogs, this drug has been prescribed for MVD-affected dogs already in heart failure if pulmonary hypertension is also detected. Pulmonary arterial hypertension (PH or PAH), as a complication of mitral valve disease, is higher diastolic or systolic pulmonary arterial pressure than normal pressure. This high pressure may lead to increased right ventricular pressure and right atrial chamber enlargement, leading to possible right side heart failure.
The 2020 ACVIM Consensus Statement of guidelines for the diagnosis, classification, treatment, and monitoring of PH in dogs cautions that sildenafil or any other PDE5i pulmonary hypertension drug should not be prescribed as a first line treatment of PH secondary to MVD. Their reasoning is that, dogs with PH secondary to MVD have postcapillary PH. Postcapillary PH is a term given to left atrial (LA) or left ventricular filling pressure, which occurs most often in dogs with MVD that have increased LA pressure. Pimobendan has been found to lower LA pressure and thereby treat postcapillary PH. If, however, preliminary treatment with pimobendan and other MVD drugs does not relieve the PH, sildenafil may be added, and especially if the PH increases beyond the mild or moderate stages.
In a March 2017 report of a 180-day study of 30 MVD-affected dogs (none were cavaliers) in Stage B1 or Stage B2 (non-symptomatic), a team of Thailand investigators treated half of the group with sildenafil (Viagra, Revatio) (a/k/a sildenifil) versus a control group. They found:
• The stroke volume (the volume of blood pumped from the left ventricle per beat) at day 30 was significantly higher in the sildenafil group
• The LA/Ao (left atrial-to-aortic ratio) and the MR jet area were significantly lower beginning at day 30, day 90, and day 180.
• The 2D-LA (2-dimentional left atrium size) was significantly lower at day 90 when compared with control group.
• The differences of NTproBNP (a natriuretic peptides test biomarker in cardiac disease) from baseline were significantly lower when compared with control group at the same timepoint, day 90 and day 180. (Click here for details about natriuretic peptides tests.)
They concluded:
"In conclusion, this study suggested that long-term treatment with sildenafil prevented aggravation of disease progression as suggested by several echocardiographic indices (i.e. SV, LA/Ao, MR jet area, 2D-LA) and reduced NTproBNP level at the indicated timepoints in dogs with asymptomatic mitral valve degeneration."
In a July 2017 article, the same group of Thai researchers compared sildenafil (Viagra, Revatio) to enalapril (an ACE-inhibitor) in their effectiveness in increasing heart rate variability (HRV) in MVD-affected dogs in ACVIM Stage B (pre-heart failure). Thirty-four MVD-affected dogs and thirteen healthy dogs participated, divided into groups of control, sildenafil, and enalapril. At the outset, they noted that MVD degeneration causes an imbalance of sympathovagal* activity, which results in poor cardiac outcomes. The results showed that MVD-affected dogs had significant higher heart rates (HR), systemic blood pressures, and significant decreased HRV than the healthy control dogs. After treatment with sildenafil for 90 days, both time- and frequency-domain parameters were significantly increased when compared with control and enalapril groups. This study demonstrated that sildenafil improves HRV in Stage B dogs, and that enalapril did not. They concluded that this result suggests that sildenafil should be used in MVD-affected dogs to restore the sympathovagal balance.
* Changes in the dog's heart rate are tied to changes in cardiac output. Heart rate variability (HRV) reflects variations in the time periods between heart beats. Generally, a low HRV indicates that the dog is under stress, which may be due to exercise, psychological events, or other internal or external events. A higher HRV usually means that the dog has a strong ability to tolerate stress or is strongly recovering from prior accumulated stress. A reduction in HRV in MVD-affected dogs reflects a "sympathovagal imbalance", which has been identified as a risk factor for sudden cardiac death. "Sympathovagal" refers to an interaction between the dog's sympathetic nervous system and its vagus nerve. HRVs reflect the output of the heart as regulated by the sympathetic nervous system.
Alternative drugs to sildenafil include tadalafil (Cialis), beraprost, bosentan (Tracleer), and varenafil (Levitra). For more information about treating pulmonary hypertension in MVD-affected dogs, go to our discussion under medications for Stage C.
RETURN TO TOP
--- diuretics in Stage B2
BOTTOM LINE: Diuretics (e.g. furosemide, Lasix) have not been shown to be appropriate if the dog has not reached the stage of heart failure (Stage C).
Diuretics are intended to treat fluid buildup in the lungs, which is pulmonary edema, a symptom of left-sided congestive heart failure (Stage C). Stage B2 does not include signs such as fluid retention in the lungs. Furthermore, we know of no published veterinary research supporting administering diuretics to MVD-affected dogs in Stage B2. As board certified veterinary cardiologist Dr. Janet Olson states in her December 2016 article about diuretics, "Therefore, it is necessary to take thoracic radiographs to confirm the presence of pulmonary edema prior to starting lasix."
Nevertheless, some cardiologists hypothesize that administering a low dose of diuretic to a Stage B2 dog if enlargement of the left atrium (LA) is impinging upon the brochus or trachea and causing coughing, may decrease pressure in the LA and also delay the onset of heart failure, notwithstanding the lack of any published veterinary literature with any findings or conclusions supporting such pre-CHF treatment with diuretics, and also the known damage that diuretics can do to dogs' kidneys and liver. See this article, "Should MVD-Affected Dogs Start Furosemide Treatment Before Congestive Heart Failure?", on our Blog webpage.
To learn more about diuretics, go to our discussion of them in our Treatment - Stage C section.
Our Blog: "Should MVD-Affected Dogs Start Furosemide Treatment Before Congestive Heart Failure?"
RETURN TO TOP
--- ACE-inhibitors in Stage B2
BOTTOM LINE: ACE-inhibitors are not effective in treating MVD-affected cavalier King Charles spaniels before heart failure (Stage C).
ACE-inhibitors -- enalapril maleate (Enacard, Vasotec, Prilenal, Envas), benazepril hydrochloride (Lotensin, Fortekor, Banacep Vet, Benefortin, Benazecare, VetACE, Prilben, Nelio), imidapril (Tanatril), ramipril (Altace, Tritace, Vasotop), captopril (Capoten), fosinopril, lisinopril (Zestril, Prinivil, Qbrelis), quinapril (Accupril), alacepril, temocapril (Acecol), and zofenopril -- may also be prescribed by some cardiologists. ACE-inhibitors (ACE-I) are intended to block the angiotensin converting enzyme, which is necessary to produce a substance that causes blood vessels to tighten. So, ACE-I serve to relax the blood vessels, thereby lowering the blood pressure and increasing the supply of blood and oxygen to the heart. However, studies have shown only a low concentration of angiotensin II receptors in dogs' affected mitral valves. Therefore, the 2009 ACVIM Consensus Statement has not endorsed ACE-I therapy of MVD dogs at Stage B2 MVD. Further, ACE-inhibitors have not been approved for veterinary medicine for MVD dogs in Stage B2 by regulating authorities. However, oddly enough, a majority of the ten members of the 2009 ACVIM Consensus Statement panel did recommend starting an ACE-inhibitor at Stage B2 (although their reasoning was not included in the Consensus Statement).
The 2009 Consensus Statement was revised by the 2019 Consensus Statement. Among the changes are that ACE-I therapy still has not been approved, but this time the split between the ten cardiologists participating in the report is 5 for and 5 against.
An additional reason for not prescribing ACE-inhibitors prior to heart failure is a 2002 Scandinavian study (the "Scandinavian Veterinary Enalapril Prevention [SVEP] Trial") of 229 asymptomatic cavalier King Charles spaniels with mild MVD murmurs, which has has shown that ACE-inhibitors had no significant affect upon the time from the initiation of ACE-I therapy to the point of heart failure (HF). The authors stated:
"[T]his study failed to show any preventive effect of enalapril for developing CHF. ... Indeed, the finding in this study, ie, that enalapril did not prevent CHF regardless of whether the dogs had cardiomegaly or not at the time of initiation of therapy, speaks against the possibility that ACE inhibition prevents progression of dilatation. Accordingly, the practice of starting ACE inhibitor treatment in dogs with MVD as soon as cardiomegaly is evident, as is commonly recommended and used in clinical practice, is not supported by the present study. ... In conclusion, long-term treatment with ACE inhibition in asymptomatic dogs with MVD does not delay the onset of CHF regardless of whether cardiomegaly is present at initiation of therapy or not. " (Emphasis added.)
A 2007 study (the "VETPROOF Trial"), sponsored by an ACE-inhibitor manufacturer and involving 124 dogs of several breeds (including only 10 cavaliers), showed that enalapril given to dogs with only mild MVD murmurs and some enlargement of the heart but which otherwise are symptomless, "modestly delayed" the onset of HF. (Also, see below for discussion of ACE-inhibitors' adverse side effects.) Further, in a 2013 study by Thai graduate students, of twenty dogs (none CKCS) in Stage B2, they found that ramipril did not affect cardiac chamber size, mitral regurgitation severity and systolic function assessed by echocardiography in 91-day period of treatment.
This chart, prepared by veterinary cardiologist Dr. Niek Beijerink, compares the results of the two ACE-inhibitor studies. The 2002 SVEP study of only cavaliers is summarized on the left, and the 2007 VETPROOF study is on the right. He concluded from these results that no ACE-inhibitors should be given prior to heart failure -- Stage C (see below). Other researcher-cardiologists, including Drs. Mark Oyama and Adrian Boswood, have reached the same conclusion that ACE-Is at Stage B2 is not appropriate. In a 2012 presentation, Dr. Oyama stated that not giving an ACE-inhibitor to Stage B2 cavalier King Charles spaniels was "a slam dunk" decision, in view of the SVEP trial of 229 cavaliers, noted above.
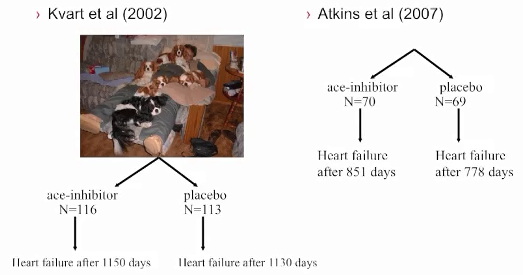
Benazepril likewise has been found to not postpone the onset of CHF in cavaliers. In this July 2008 article, this drug had no beneficial effect on CKCSs with Stage B MVD. It did not delay the onset of heart failure in the cavaliers in that study.*
* Interestingly, the findings in the June 2008 article also did not demonstrate any differences in the time to onset of CHF in any other dogs in the study, although oddly, the report's conclusion seems to contradict that express finding. The finding is: "No differences in the time to onset of CHF were found between the BNZ [benazepril] and UT groups in the whole study population or in the OB [other breed] and CKC [Cavalier King Charles spaniels] populations." The contradictory conclusion is: "BNZ had beneficial effects in asymptomatic dogs other than CKC and KC [King Charles spaniels] affected by MVD with moderate-to-severe MR." So, go figure.
In an October 2017 article, researchers reported that a polymorphism of an ACE gene, intron 16, is most commonly found in the CKCS and that MVD-affected dogs with that mutation tend to have significantly lower ACE activity. In a December 2017 article, the same team of researchers studied 73 cavalier King Charles spaniels and reported finding that:
"The CKCS appears to have a high prevalence of the ACE variant. Dogs with the ACE variant had lower levels of ACE activity even in more advanced mitral valve disease than dogs without the variant."
They concluded that the impact of their finding "on the need for ACE-I in dogs with the polymorphism and heart disease deserves further study."
In a July 2021 article, this same team identified studied the gene for the ACE enzyme -- canine chromosome 9:11507816:G > A -- in 497 dogs of 54 breeds, including 13 cavaliers, to determine the prevalance of a variant of that gene which prevents ACE-inhibitors from suppressing the RAAS and instead allows aldosterone breakthrough. The ACE gene variant was found in 32 of the 54 breeds. The variant was greatest five breeds, including the CKCS with a prevalence of 85%. The other four breeds were the Irish wolfhound (95%), Dachshunds (90%), great Danes (84%), and bull mastiffs (58%).
Nevertheless, Dr. John Bongura reported in 2010 that out of 100 board certified veterinary cardiologists surveyed, nearly 70% indicated that an ACE-inhibitor was warranted for dogs in Stage B2, notwithstanding the lack of any research reports supporting their conclusions.
ACE Inhibitors -- More Pluses Or Minuses?
In studies, ACE-inhibitor enalapril has improved survival by more than 100%, and reduced pulmonary edema and improved quality of life scores, for dogs in congestive heart failure (CHF). Studies also found that exercise capacity was also improved in dogs with experimental mitral regurgitation. Benazepril also has been shown to improve survival, and imidapril was shown to be equal to enalapril in survival studies of dogs in heart failure due to mitral regurgitation.
However, ACE-inhibitors may have serious side effects. They can cause severe renal insufficiency, and the kidneys should be monitored carefully when using these drugs. A baseline renal panel should be conducted before treatment is initiated and again five to ten days later. Adverse reactions to the drug typically occur within three to five days of therapy initiation.
Benazepril (Lotensin, Fortekor, Benefortin, VetACE) is reported to be slightly less harsh on the kidneys than is enalapril maleate (Enacard, Vasotec) or ramipril (Altace, Tritace, Vasotop). Also, ACE-inhibitors traditionally are used for the treatment of high blood pressure. However, not all veterinary cardiologists check a cavalier's blood pressure before prescribing the drug. Unless the dog's blood pressure is high, the use of an ACE-inhibiting drug could dangerously lower its blood pressure.
The use of ACE inhibitors combined with extreme salt (sodium) restriction may contribute to renal dysfunction by activating the renin-angiotensin aldosterone system (RAAS). Therefore, some cardiologists recommend only moderate salt restricted diets when prescribing ACE inhibitors.
Other side effects include the accumulation of toxins which can damage the liver, anorexia or loss of appetite, vomiting, azotemia -- elevation of blood urea nitrogen (BUN) -- and the development of a dry cough due to the accumulation of bradykinin. Since a dry, hacking cough is a frequent symptom of progressing MVD, this side effect of the drug could be confused with the worsening of the disease.
In a February 2020 article, Dr. Mark Rishniw carefully dissected all five drug-company-funded studies of the ACE-inhibitor enalapril (the IMPROVE, COVE, LIVE, SVEP, and VETPROOF studies) to determine whether the drug would delay the onset of cardiac death in MVD-affected dogs in heart failure (CHF) or would delay the onset of CHF in pre-clinical MVD-affected dogs (i.e., in Stages B1 or B2). While the conclusions of some of the studies were muddled by drug-company interference, Dr. Rishniw's analysis of the underlying data showed that ACE-inhibitors have not been scientifically shown to delay the onset of either CHF or cardiac death due to MVD. He goes on to determine that:
"In retrospect, it became clear that the veterinary community was hoodwinked into believing that ACE-I were the medical cure for CHF dogs because of corporate marketing decisions."
"So, what does all this mean? My interpretation of all this literature suggests that ACE-I have no place in managing mitral valve disease, whether in dogs with CHF or dogs with subclinical disease."
Notwithstanding the lack of effectiveness of ACE-inhibitors in delaying the onset of CHF, alacepril may be effective in reducing or eliminating the cardiac-related cough in Stage B2 dogs. In an August 2018 article, a team of Japanese researchers tested the angiotensin-converting enzyme inhibitor (ACE-inhibitor) alacepril on 36 dogs, including four cavalier King Charles spaniels, which were in Stage B2 of mitral valve disease (having an MVD-murmur and an enlarged heart but prior to heart failure) and all displaying an MVD-related cough. They primarily were testing the cough-suppression efficacy of alacepril over a period of four weeks. They report finding that alacepril resolved or lessened the cough in 20 (55.6%) of the dogs and had no cough-suppressant effect upon the remaining 16 (44.4%). They also found that alacepril reduced LVIDDN in the effective group in the present study, noting that LVIDDN is known as an indicator of left ventricle enlargement in dogs. These invsetigators observe that alacepril is among a sub-group of ACE-inhibitors (including captopril and zofenopril) which contain sulfhydryl, which may confer properties additional to ACE inhibition, which may explain the cough-suppressant qualities.
Studies currently are planned to determine if aldosterone receptor blockers (AFBs) including Losartan potassium (Cozaar), telmisartan (Micardis), valsartan (Diovan), sacubitril/valsartan (Entresto), and direct renin inhibitors (DRIs) including aliskiren (Tekturna, Rasilez), will significantly lower the incidence of ABT than will treatment with ACE-inhibitors.
In an August 2018 article, a team of Auburn University researchers examined the effectiveness of an aldosterone receptor blocker, sacubitril/valsartan (S/V), in 7 dogs diagnosed with mitral valve disease (MVD), compared to 6 MVD-affected dogs in the placebo group. They report finding that S/V is effective at lowering the urine concentration of aldosterone and that it was safe, with no deleterious effects on BUN, creatinine, and electrolyte concentrations, or systolic arterial pressure.
A baseline renal panel should be conducted before treatment is initiated and again three to five days later. Adverse reactions to the drug typically occur within three to five days of therapy initiation.
--- natural alternatives to ACE-inhibitors
A natural supplement as an alternative to ACE-inhibitors is a combination of active fish petides, including LKPNM, from the bonito fish (Sarda orientalis), such as Vasotensin, manufactured by Metagenics, Inc., and PeptACE by Natural Factors. Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained in TCM. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
Other Chinese herbal alternatives include Salvia Shou Wu, a Seven Forests patented supplement which consists of Salvia extract, and several other herbs and flowers. Salvia Shou Wu encourages blood circulation.
RETURN TO TOP
--- spironolactone, aldosterone antagonist in Stage B2
Spironolactone (Aldactone, Prilactone, CaroSpir), a synthetic 17-lactone drug, is a mild diuretic. However, there are no published studies of the efficacy of spironolactone as a diuretic in dogs. It also is an aldosterone antagonist (or mineralocorticoid receptor blocker -- MRB). Aldosterone is a hormone in the mineralocorticoids group. Its function is to regulate blood pressure and sodium potassium levels. High levels of this hormone may have harmful effects in the heart, including as mechanism of cardiac remodeling, meaning enlargement.
There are no peer-reviewed veterinary studies recommending beginning spironolactone in Stage B2. In a July 2017 article by a team of UK investigators, they report the inconclusive results of a pilot study of 25 dogs (13 treated with spironolactone and 12 with placebo). All of the dogs were MVD-affected and in Stage B2. None of them were being treated with any other drugs. Just about the only thing the study results confirm is their statement that the drug's "efficacy in preclinical MMVD is unknown."
Spironolactone is known as a potassium-sparing diuretic because, unlike some other diuretics, it does not cause the loss of potassium. However, it reportedly may lead to excessively high, life-threatening levels of potassium in the dog's blood, particularly when combined with ACE inhibitors. Some veterinary cardiologists recommend that potassium levels be carefully monitored when using spironolactone in combination with ACE inhibitors by drawing blood at regular intervals until it is evident that the potassium level is or is not going to be a problem.
In a 2013 study of the possible increased risk of adverse events for dogs taking spironolactone in addition to conventional therapies, the researchers concluded that dogs with heart failure receiving spironolactone in addition to conventional treatment are not at a higher risk for any adverse events, death caused by cardiac disease, renal disease, or both, hyperkalemia, or azotemia. The study was funded by the manufacturer of Prilactone. See also this May 2016 review of the use of spironolactone as a diuretic.
Cardalis is a tablet which combines spironolactone with the ACE-inhibitor benazepril. Aldactazide is a combination of spironolactone and hydrochlorothiazide, which inhibits the activity of the hormone aldosterone.
 The DELAY Study: In 2010, a team of Italian cardiologists, led by
Dr. Michele
Borgarelli (now at VA-MD Regional College of Veterinary Medicine in
Virginia, USA), began the DELAY Study to determine if giving
combined doses of spironolactone and benazepril can delay the
onset of symptoms of heart failure in dogs with MVD. The results of the study
were reported in
a January 2020 article, and they were mixed. A total of 183 dogs
participated, including 34 cavaliers (19%), the highest number of
purebreds. Mixed breeds totaled 72 (40%). The dogs were divided into a
treatment group (87 dogs) and control group (92 dogs) which received a
placebo and no medication. At the end of the study, 42 dogs in the
treatment group and 33 in the control group remained (most of the
drop-outs opting to switch treatment to pimobendan). Of those, the
median time to reaching heart failiure in the treatment group was 902
days, and the median time in the placebo group was 1,139 days, a
difference of 237 days, roughly 8 months. However, the treatment group's
heart measurements showed that treatment could reduce -- and in some
cases even reverse -- heart enlargement and reduce NT-proBNP. They
concluded:
The DELAY Study: In 2010, a team of Italian cardiologists, led by
Dr. Michele
Borgarelli (now at VA-MD Regional College of Veterinary Medicine in
Virginia, USA), began the DELAY Study to determine if giving
combined doses of spironolactone and benazepril can delay the
onset of symptoms of heart failure in dogs with MVD. The results of the study
were reported in
a January 2020 article, and they were mixed. A total of 183 dogs
participated, including 34 cavaliers (19%), the highest number of
purebreds. Mixed breeds totaled 72 (40%). The dogs were divided into a
treatment group (87 dogs) and control group (92 dogs) which received a
placebo and no medication. At the end of the study, 42 dogs in the
treatment group and 33 in the control group remained (most of the
drop-outs opting to switch treatment to pimobendan). Of those, the
median time to reaching heart failiure in the treatment group was 902
days, and the median time in the placebo group was 1,139 days, a
difference of 237 days, roughly 8 months. However, the treatment group's
heart measurements showed that treatment could reduce -- and in some
cases even reverse -- heart enlargement and reduce NT-proBNP. They
concluded:
"This study failed to demonstrate a prolongation of the asymptomatic phase of the disease in this population of dogs. Nevertheless, the treatment appears effective in reducing or even reverse cardiac remodeling as indicated by various cardiac imaging parameters and NT-proBNP concentrations. Chronic administration of combined spironolactone and benazepril treatment in dogs with pre-clinical MMVD and cardiac enlargement is safe and well tolerated. The significant effect of this treatment on secondary end-points could be of clinical relevance and merits further study."
To read more about spironolactone, go to our discussion of it in our Treatment - Stage C section.
RETURN TO TOP
--- angiotensin receptor blockers (ARBs) in Stage B2
Angiotensin receptor blockers (ARBs) are drugs intended to block activation of the renin-angiotensis-aldosterone system (RAAS) due to RAAS' known contribution to the development of CHF in MVD-affected dogs. ARBs include
•
telmisartan (Micardis)
•
sacubitril/valsartan
(Entresto, Vymada)
• valsartan (Diovan)
Studies currently are underway or planned to determine if some of these ARBs will significantly lower the incidence of ABT than will treatment with ACE-inhibitors.
Telmisartan (Micardis): A typical initial dosage is 1 mg/kg orally every day. Blood serum contents of blood urea nitrogen (BUN), creatinine, sodium, potassium, and chloride should be checked before beginning and periodically afterwards. Telmisartan and other ARBs may have these uncommon side effects: gastrointestinal upset, weakness, lethargy, azotemia, hyperkalemia (excessively high potassium level), and hypotension (low blood pressure).
In an October 2018 article, Japanese researchers reported that the ARB telmisartan did not suppress RAAS activation in five healthy laboratory beagles.
In a December 2020 article, University of Pennsylvania researchers compared the effectiveness of angiotensin converting enzyme inhibitors (ACE-I) -- enalapril and benazepril -- with the angiotensin receptor blocker (ARB) telmisartan in offsetting the activatation of the renin-angiotensin-aldosterone-system (RAAS) in the kidneys of dogs in Stage B2 of mitral valve disease. Eight MVD-affected dogs were included in the study, all diagnosed with Stage B2 of MVD, including two cavalier King Charles spaniels (25%). They report finding that telmisartan treatment resulted in higher activity of favorable angiotensin peptide (AP) concentrations, as compared to ACE-I treatment. They concluded that:
"Treatment with ARBs, such as telmisartan, or combined ARB and ACE2 [angiotensin converting enzyme 2] treatment represents a potential treatment strategy for dogs with DMVD and warrants additional preclinical study."
Sacubitril/valsartan (Entresto, Sacufox V, Vymada, LCZ696): Sacubitril/valsartan (Entresto, Sacufox V, Vymada, LCZ696) is a combination of valsartan, an angiotensin II receptor blocker (ARB), which prevents vasoconstriction thereby lowering blood pressure and improving blood flow. and sacubitril, a neutral endopeptidase (neprilysin -- NEP) blocker, that prevents the breakdown of natriuretic peptides, which helps lower blood pressure by reducing sodium levels. It is intended to promote diuresis and natriuresis and counteract the effects of RAAS. It is being used as an alternative to ACE-inhibitors in Stage C and D MVD-affected dogs. A dosage ranges from 5 to 10 mg/kg to 20 mg/kg orally every 12 hours.
In an August 2018 article, a team of Auburn University researchers examined the effectiveness of sacubitril/valsartan (S/V) in 7 dogs diagnosed with mitral valve disease (MVD), compared to 6 MVD-affected dogs in the placebo group. They report finding that S/V is effective at lowering the urine concentration of aldosterone and that it was safe, with no deleterious effects on BUN, creatinine, and electrolyte concentrations, or systolic arterial pressure.
In a February 2019 article, the investigators compared the effects of sacubitril/valsartan versus just valsartan, and versus benazepril, on the dynamics of the renin-angiotensin-aldosterone system (RAAS) and the natriuretic peptides (NP) system in 18 laboratory beagle dogs after activating their RAAS with a low-salt diet over 15 days. They reported:
"In conclusion, the ARNI sacubitril/valsartan reduced ALD, a known risk factor of CV mortality, and enhanced the NP system via cGMPmediated pathways in a low-sodium diet model of RAAS activation. The results presented herein provide further evidence that the effects on the renin cascade extend to reduced ALD levels beyond that achieved with RAAS blockade alone. These positive findings in dogs also suggest that sacubitril/valsartan is a promising pharmacological candidate for increased survival in canine cardiovascular diseases."
In a November 2020 article, 7 dogs in which cardiorenal syndrome (CRS) was induced, resulting in heart failure and renal dysfunction, were treated with S/V for 3 months and compared to 7 other dogs in a control group. The investigators reported that the S/V improved left ventricle systolic function and mitochondrial function and decreased biomakers of heart and kidney injury.
In a July 2021 article, Thai investigators compared sacubitril/valsartan with the ACE-inhibitor ramipril in treating dogs diagnosed with Stage C (heart failure) due to MVD, along with both pimobendan and the diuretic furosemide. Twenty-one dogs -- none being cavaliers -- were divided into the S/V group (11 dogs) and the ramipril group (10 dogs) for four weeks. The investigators report that short-term administration of SV in dogs with MVD stage C resulted in a greater extent of reverse myocardial remodeling of both the left atrium and the left ventricle than in the ramipril group, as indicated by several echocardiographic parameters. They concluded:
"The current study suggested that the short-term effects of SV can reverse myocardial remodeling, as inferred from several echocardiographic indices (i.e., the reduction in LA/Ao, LVIDDN, EDVI and ESVI) in dogs with MMVD stage C. These findings would support the use of SV in clinically symptomatic heart failure in dogs."
However, they warn of a few limitations: (a) a very small sample size, with a much larger sample size needed to confirm the findings; (b) the study was for a very short term -- four weeks -- and a longer term study should be conducted; (c) the results thus far "do not suggest that ACEi should be replaced with S/V in dogs with MMVD stage C."
In a June 2024 article, Japanese resaerchers studied the effects of Entresto on the kidneys of 5 healthy laboratory dogs. They were given oral doses of 20 mg/kg. twice a day for 4 weeks. Kidney blood flow (renal hemocynamics) was assessed before the first dose and then on days 7 and 28. They report finding that Entresto "may enhance renal haemodynamics in healthy dogs." Translated, this means Entresto may improve the blood flow through the kidneys.
At the June 2025 ACVIM Forum, Dr. Justin Carlson presented a lecture, "Beyond the Guidelines: Sacubitril/Valsartan (ARNi) (Entresto)", in which he reported on 49 case studies of MVD-affected dogs in late Stage C or Stage D ("pretty bad cases that I thought were not going to do very well"), 7 of which were cavalier King Charles spaniels (and 7 Maltese and 6 Yorkies). Among those 49 patients in which he substituted Entresto (sacubitril/valsartan - S/V) for an ACE-inhibitor (such as benazepril or enalapril) and spironolactone, their mean survival time was 474 days, with a median of 381 days, and an overall survival range from 152 to 1,989 days. He compared those results to those of the VALVE Study trial and the QUEST Study. In the VALVE Study of Stage C dogs (in which the ACE-inhibitor ramipril was added to the combination of furosemide and pimobendan), the median survival time was only 214 days. In the QUEST Study (in which Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan alone. He emphasized that the patients in his group of 49 dogs being treated with Entresto were late Stage C or Stage D dogs, meaning that they were in far worse shape than the Stage C dogs just developing heart failure which were the subjects of the VALVE and QUEST studies.
Dr. Carlson said that the owners of 39 of his 49 patients (80%) reported their dogs had noticeably improved energy levels. The owners of 28 of the dogs (57%) reported that their dogs were coughing less. He found that "in a lot of these dogs", the S/V allowed for the safe reduction of diuretics, and in "a handful of these dogs", he has since stopped any diuretic medications, and "they are doing great." He reported that, over time, there was "a statistically significant difference" in the sizes of the left ventricle and left atrial ratio. He found in some dogs that over time their left atriums were back to normal in size.
As for adverse effects, he had only 2 of the 49 dogs with gastrointestinal distress. As for renal values, only one patient had significant worsening renal values, with 3 having increased azotemia in 4 months and 5 having significantly decreased renal values after 4 months. Regarding survival times, the mean survival time was 474 days, with a median of 381 days , and a range from 152 to 1,989 days. He pointed out that in the VALVE Study trial, in which an ACE-inhibitor (ramipril) was added to the combination of furosemide and pimobendan, the median survival time was only 214 days, and in the QUEST Study (in which the Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan. He emphasized that the patients in his group of dogs being treated with Entresto were Stage D dogs.
Dr. Carlson recommends "up-titration" of dosages of S/V, meaning starting at a low dosage and then increasing it, over 2 to 4 weeks, depending upon the dog's response and the blood test results. He stops both ACE-inhibitors and spironolactone when he starts the S/V, waiting 36 hours. His recommended dosage range in 15-30 mg/kg twice a day.
In a paper presented at the 2025 ACVIM Forum, cardiologists Ryan Fries, Elizabeth Malcolm, Sonya Gordon, and Justin Carlson wrote:
"When utilizing Entresto® in a group of canine patients (n=49) with congestive heart failure secondary to myxomatous mitral valvular disease or dilated cardiomyopathy, Entresto was well tolerated, with fewer than 5% of patients having undesirable side effects when titrated appropriately. While a FETCH questionnaire was not completed on patients, there was an overwhelming owner-noted improvement to the patients' quality of life when optimal dosing of Entresto was achieved based on owner report history during follow-up. Up-titration of the medication was done over the span of 1-6 weeks in most patients, with some patients requiring an extended up-titration over multiple months. Survival, echocardiographic, and dose-response data will be presented in a future manuscript. Based on the encouraging data from this cohort of patients, the optimal dosing, timing of up-titration, and ideal start time in patient disease stage warrant further study in a prospective manner."
The U.S. Food & Drug Administration (FDA) approved S/V for humans in 2015 to redue the risk of cardiac death in patients with chronic heart failure. Valsartan (Diovan) alone had been approved by the FDA in 2002 for treating humans of heart failure. FDA approval of S/V for humans was based upon a study (PARADIGM-HF) comparing it to enalapril in 8,442 patients with symptomatic chronic heart failure and systolic dysfunction. Studies of humans recommend up-titration from the lowest dose to the highest tolerable dose over periods of 2 to 4 weeks. It is for these reasons that veterinary cardiologists in general prefer to wait until the MVD-affected dog is well into heart failure (Stage C) or even in Stage D before starting S/V, and for up-titrating the dosages.
 The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
Reported side effects of Entresto include:
• Diarrhea
• Vomiting
• Drop in blood pressure
• Weakness
• Difficulty standing
• Kidney issues
There is a Facebook group, Entresto for dogs with CHF/MVD - sharing stories and what we have learned, which consists of hundreds of members who discuss their dogs' experiences with Entresto.
RETURN TO TOP
--- alpha & beta blockers in Stage B2
Carvedilol (Coreg), and bisoprolol, also are being prescribed for Stage B2 MVD by some cardiologists. They are non-selective beta-and alpha-blockers with anti-oxidant effects -- also known as beta- (B-) adrenergic receptor antagonists (BARA) -- which reduce the heart's rate and the force of its contraction, thereby reducing the work of the heart. Also, in a 2009 study, it has been suggested that BARA have the potential to slow the progression of the mitral valve's degeneration by interfering with the serotonin signaling pathway -- a possible major factor in MVD progression -- and by reducing the "wear and tear" of the valve by reducing the pressure differences between the left ventricle and atrium. In a 2012 report, researchers studied the effect of carvedilol in treating cavaliers with Stage B2 MVD and found no adverse effects and median survival of 48.5 months. See also this January 2015 report regarding Carvedilol's effect upon hypertension.
Phenoxybenzamine ( Dibenzyline) is another alpha-blocker, with vasodilator properties.
A less expensive alternative beta-blocker is atenolol (Tenormin, Tenoretic). However, atenolol lacks the vasodilatory and antioxidant properties of carvedilol. Other beta blockers include metoprolol (Lopressor, Toprol).
Nicorandil (Ikorel, Angedil, Dancor, Nikoran-PCA, Aprior, Nitorubin, Sigmart), is an alternative to beta-blockers and/or calcium antagonists. It is a potassium channel activator with arterial vasodilator and venodilating properties. It increases blood flow through the heart, improves the heart's blood and oxygen supplies, and reduces its workload. Nicorandil is not available in the United States. See this January 2011 article on nicorandil.
RETURN TO TOP
--- D-Ribose in Stage B2
Read about D-Ribose here as a supplement to strengthen the heart muscle and otherwise energize MVD-affected dogs with enlarged left atriums and/or left ventricles.
RETURN TO TOP
--- other drugs for treating Stage B2
Ivabradine is a selective sinus node If Channel (funny channel) inhibitor which may be used to lower and maintain heart rate without affecting cardiac function. In a November 2017 abstract, researchers report finding that ivabradine lowers the heart rate in dogs, with no clinical discernable side effect, and therefore is safe to use in dogs with mitral regurgitation.
In a May 2018 study of seven laboratory Beagles with naturally-occuring asymptomatic MVD (ACVIM Stage B), the researchers reported that a single oral dose of ivabradine for dogs with asymptomatic MVD reduced both the dogs' heart rate (HR) and the hearts' consumption of oxygen (MVO2) without significantly affecting blood pressure. They concluded that ivabradine may be promising for management of an elevated HR in asymptomatic MVD-affected dogs. In a September 2018 article, the same team of Thai veterinary researchers conducted a long term (3 months) study of oral doses of ivabradine in four MVD-affected Beagles with heart enlargement (Stage B2). They evaluated the dogs'heart rate (HR), blood pressure (BP), myocardial oxygen consumption (MVO2), and heart rate variability (HRV). They report finding that "the results revealed that chronic administration of ivabradine significantly decreased HR, BP, and RPP without adverse effects. All indices of time- and frequency- domain of HRV at M3 were significantly increased when compared with baseline values)." They conclude that "The findings of this study imply that long-term treatment with ivabradine at a dose of 1.0 mg/kg twice daily in dogs with asymptomatic DMVD stage B2 decreased the HR, BP, MVO2 and improves HRV. This makes ivabradine potentially promising for management of elevated HR and impaired HRV in asymptomatic dogs with DMVD stage B2."
In a January 2026 article, Thai researchers investigated the effect of long-term oral ivabradine on heart rate (HR), haemodynamics, cardiac function, and apoptosis (programmed cell death) in dogs with Stage B2 of MVD, meaning having a murmur and left-side heart enlargement but no fluid in the lungs (CHF). They treated 4 laboratory beagles in Stage B2 with oral ivabradine for 3 months. They found that the drug decreased the HR by 30.4% without significantly affecting systemic or pulmonary blood pressures, vascular resistance, or systolic/diastolic functions of the heart. They concluded that regular treatment with ivabradine lowered HR and reduced cardiac remodeling and apoptosis without impairing cardiac function in those dogs in Stage B2 of MVD.
RETURN TO TOP
--- Bronchial dilators for coughing
MVD-affected dogs with an enlarged left atrium may develop a periodic, odd-sounding cough which cardiologists often describe as a "cardiac cough".
Some cardiologists may prescribe a bronchial dilator (bronchodilator), such as a methylxanthine, for example, aminophylline, millophyline, oxtriphylline, theophylline* (Corvental, Nuelin, Apo-Theo-LA, Theo-Dur), or terbutaline (Brethine, Bricanyl) which are human grade prescription medications which relax and open air passages in the lungs, making breathing easier.** A narcotic, hydrocodone bitartrate with homatropine MBr or guaifenesin (Hycodan, Hydromet, Tussigon, Mycodone), or butorphanol tartrate (Torbutrol, Stadol, Torbugesic) may be prescribed to suppress the coughing by affecting the brain's cough centers.
* Theophylline is a
PDE inhibitor which should not be given concurrently with pimobendan, which
is another PDE inhibitor, unless the combination of those two drugs is
carefully balanced.
** Fluoroquinolone antibiotics should not be given concurrently with any
methylxanthines.
The cough could be due to a combination of factors, which include the enlarged left atrium of the heart pressing against and compressing the left mainstem bronchus, but more likely to airway disorders independent of any relationship with the heart. It may even cause the trachea to collapse*.
* Trachea collapse also may be due to Brachycephalic airway obstruction syndrome (BAOS).
In a March 2012 article, USA researchers found no association between left atrial enlargement (LAE) and bronchial collapse in MVD-affected dogs with moderate-to-severe LAE and chronic coughing, but they did find that airway inflammation is common in dogs with airway collapse.
In a January 2019 article, a pair of experts concluded that a cough in the absence of rapid and labored breathing would indicate that it is due mainly to a respiratory disease rather than a cardiac disease. Coughing is a hallmark sign of bronchitis. Dogs with severe pulmonary edema can cough but coughing is much more common with primary lung disease. By severe pulmonary edema, they mean that the fluid has completely filled the lungs and also has started to fill the upper airway passages, as well. (The x-ray below shows the heart's enlarged left atrium impinging upon the left main bronchus.).
One of the most common -- and potentially dangerous -- misjudgements some veterinarians tend to make is to assume that such a cough indicates the onset of CHF and the need for immediate treatment with a diuretic. Cardiologist Dr. Michele Borgarelli has warned:
"It should be stressed that cough is a general clinical sign of respiratory disease and its presence in a dog with a murmur is not an indicator for starting treatment for CHF."
RETURN TO TOP
--- avoid vaccines
 Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.)
Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.)
Some veterinarians recommend that dogs with advanced mitral valve disease not be vaccinated with the usual serums, including rabies, because of possible adverse reactions which might accelerate damage to the dogs' hearts. In such cases, the veterinarians will write letters to the county licensing authorities which require periodic vaccinations, and in many instances, the counties will accept the veterinarians' letters and excuse the dogs from having to be vaccinated.
For example, Dr. Larry Glickman, veterinary immunologist at Purdue University's veterinary school, wrote regarding cavaliers:
"Our ongoing studies of dogs show that following routine vaccination, there is a significant rise in the level of antibodies dogs produce against their own tissues. Some of these antibodies have been shown to target the thyroid gland, connective tissue such as that found in the valves of the heart, red blood cells, DNA, etc. I do believe that the heart conditions in Cavalier King Charles Spaniels could be the end result of repeated immunisations by vaccines containing tissue culture contaminants that cause a progressive immune response directed at connective tissue in the heart valves. The clinical manifestations would be more pronounced in dogs that have a genetic predisposition [although] the findings should be generally applicable to all dogs regardless of their breed."
RETURN TO TOP
-- Stage C -- heart failure
- The Lasix trial
- Diuretics
- Pimobendan
- Dual therapy (diuretic and pimobendan)
- ACE-inhibitors
- Triple therapy (diuretic, pimobendan, ACE-inhibitor)
- Spironolactone
- Quadruple therapy (diuretic, pimobendan, ACE-inhibitor, spironlactone)
- Combinations in one pill
- Sacubitril/valsartan (Entresto)
- Amlodipine
- Isosorbide dinitrate (ISDN) & arteriolardilators
- Angiotensin receptor blockers (ARBs)
- Alpha & beta blockers
- Omecamtiv mecarbil
- Sildenafil & other hypertension medications
- Reducing & increasing plasma NT-proBNP
- Other drugs for treating Stage C
- Bronchial dilators for coughing
- Treating exercise intolerance and loss of muscle mass
- CoQ10 & D-Ribose and other natural alternatives
- Avoid vaccines
- Hospitalization
 Severe MVD normally involves a murmur that has become much louder.
However, the murmur can become more difficult to hear, if the heart's
deterioration has been sudden. So a Grade 6 murmur later could be downgraded
to a Grade 5, but that would not mean an improvement in the dog's condition.
Also, the dog likely will have some "clinical signs" of
heart failure, such
as difficulty breathing while at rest, and may not be
able to tolerate even minimal exercise. This is a Stage C dog, according to
the 2009 ACVIM
Consensus Statement.
Most MVD-affected dogs at Stage C are in congestive heart failure (CHF), a
common term used by cardiologists to describe dogs with pulmonary congestion
and edema.
Severe MVD normally involves a murmur that has become much louder.
However, the murmur can become more difficult to hear, if the heart's
deterioration has been sudden. So a Grade 6 murmur later could be downgraded
to a Grade 5, but that would not mean an improvement in the dog's condition.
Also, the dog likely will have some "clinical signs" of
heart failure, such
as difficulty breathing while at rest, and may not be
able to tolerate even minimal exercise. This is a Stage C dog, according to
the 2009 ACVIM
Consensus Statement.
Most MVD-affected dogs at Stage C are in congestive heart failure (CHF), a
common term used by cardiologists to describe dogs with pulmonary congestion
and edema.
Cardiologists categorize "clinical signs" of heart failure as either "congestive", meaning the production of fluids which may enter the pulmonary vein and the lungs, or "low output" or "forward heart failure", meaning that the heart is not pumping adequately to energize the dog's body. Here is a chart from Dr. Beardow's presentation showing the two classifications of clinical signs:
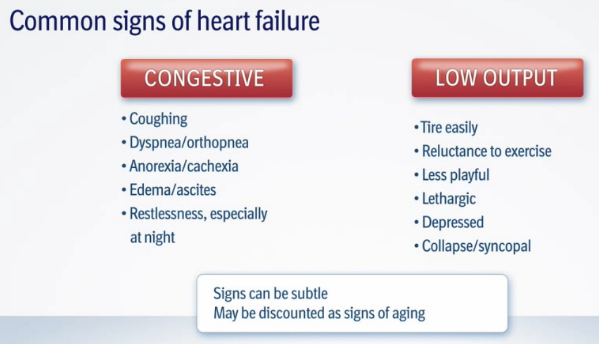
A method which cavalier owners can use to determine if and when their dog reaches the stage of heart failure is to count the dog's breaths per minute while sleeping. See our section above on Respiratory Rates for details. In an October 2012 study, researchers found that healthy adult dogs generally have a "mean" (average) sleeping respiratory rate (SRR) of less than 30 breaths per minute (BPM) and rarely exceed that rate at any time. Some cardiologists recommend that their patient's owners periodically count their dog's respiratory rate, and when the average rate starts to creep up to the high twenties, to make an appointment for the dog to be re-examined by the cardiologist to see if the dog is approaching or has reached the stage of heart failure.
The Lasix Trial: If and when the MVD-affected dog's sleeping respiratory rate (SRR) reaches or exceeds 30 BPM, that by itself does not confirm that the dog is in CHF. A technique some cardiologists may use to be certain the signs indicate heart failure is to perform the Lasix Trial. The dog will be administered a low dose of the loop diuretic Lasix (furosemide). If the dog's SRR lowers because of the Lasix dose, that will confirm that the dog has reached CHF and needs diuretic medication. If the SRR does not reduce after the Lasix trial, then the dog is not in CHF and probably is suffering from chronic bronchitis or some other respiratory disorder.
Dogs in HF with clinical signs mild enough for home therapy are classified as in Stage Cc ("c" for "chronic"). Pressure in the left atrium can be relieved by diuretics* and drugs which lower the pressure in the veins, called venodilators. Diuretics should be given by injection in severe cases. ACE-inhibitors also have venodilating effects. A typical combination of drugs to treat dogs in Stage C is called "quad-therapy" -- the diuretic furosemide, pimobendan, benazepril, and spironolactone.
* See diuretics above. In a 2012 report, researchers compared doses of torsemide and furosemide in treating dogs with stable heart failure (Stage C). They found that "torsemide is equivalent to furosemide at controlling clinical signs of CHF in dogs and is likely to achieve greater diuresis vs. furosemide." Many cardiologists will reserve the torsemide for Stage D dogs.
Dogs with severe signs of HF -- Stage Ca ("a" for "acute") -- require hospitalization for stabilization.
Stage C typically is treated with medications to manage the heart failure by alleviating the congestion, improve the cardiac output, remove the fluids from the lungs, and maintain normal blood pressure and heart rhythm. In summary, the aim of medications at Stage C is to bring the dog out of heart failure and keep the dog out of heart failure as long as possible. Eventually, initial doses of medications for Stage C dogs may not keep the dog out of heart failure, and at that point, increases in dosages and the addition or substitution of other medications may be expected.
Below we list most of the drugs that are prescribed to treat MVD-affected dogs in Stage C. A common combination of the four most typically prescribed drugs is called "the cocktail" or "quad therapy". Those drugs are: (1) a diutetic, most often furosemide; (2) pimobendan; (3) an ACE-inhibitor; and (4) spironolactone. All of them, and others, are discussed at length below here. In addition, the cardiologist most likely will recommend dog food with substantial protein, adequate calories, and moderately reduced sodium content.
Some cardiologists sub-categorize Stage C -- mild, moderate, and advanced -- based upon the dosages of drugs necessary to maintain normal sleeping respiration (consistently below 30 breaths per minute).
Dogs with tricuspid valve disease (TVD) also may reach heart failure, called right-sided heart failure. In that case, fluid will build up in the dog's abdomen and organs below the heart, such as the liver, spleen, stomach, and gastrointestinal tract, causing enlargement of those organs. The build up of fluid is called ascites or abdominal effusion. Dogs in heart failure due to TVD also are treated with diuretics, but when they no longer are sufficient to remove the fluids, the fluids may be drained directly with a catheter inserted into the abdomen. See the subsection below on TVD for details on how TVD is treated differently from MVD.
RETURN TO TOP
--- diuretics in Stage C
Diuretic treatment will be necessary at Stage C, usually in an oral tablet form. Since a dog with moderate MVD begins to retain fluid and salt, drugs which prevent fluid retention, or which increase fluid elimination, may be used. There are four classes of diuretics commonly prescribed: (a) loop diuretics, (b) aldosterone receptor antagonists, (c) thiazide diuretics, and (d) direct renin inhibitors. Each of them affect the kidneys to remove fluids and increase urine production, thereby eliminating fluids in the lungs and also decreasing blood volume and reducing blood pressure. Side effects would be that the dog is thirstier than normal, and, of course, increased urination. (Note: all of these medications also may be prescribed for Stage D, often at higher dosages.)
Over time, oral diuretics may lose their effectiveness. In a January 2025 article, UK researchers sought to determine if subcutaneous (under the skin) furosemide hypodermic injections would control signs of congestive heart failure in dogs (and cats) following failure of administering oral pills. They observed that many MVD-affected dogs develop a resistance to orally administered diuretics for a variety of reasons, including "renal distal tubular hypertrophy (nephron remodeling), activation of the sympathetic nervous system and reninangiotensin aldosterone system, decreased diuretic delivery to the kidney (e.g. hypoalbuminemia) and/or secretion into the proximal convoluted tubule (e.g. chronic kidney disease), as well as reduced gastrointestinal absorption." They provided the owners of 13 dogs, including 3 cavalier King Charles spaniels, with injectable doses of furosemide, at a median dose of 5.5 mg/Kg/day, divided into two equal doses 12 hours apart. The found that in all cases there was "a good clinical response" based upon "satisfactory control of the animal's breathing rate and effort and overall pet owner's satisfaction." They concluded:
"This study showed that furosemide administered subcutaneously appears to be an efficacious and feasible therapeutic option for providing control of signs of cardiac congestion in both dogs and cats with previous unsatisfactory response to oral diuresis."
MVD-affected dogs on high doses or combinations of diuretics should have their electrolytes and renal function monitored periodically.
RETURN TO TOP
Loop diuretics:
Loop diuretics* include:
•
furosemide
(such as Lasix, Diuride, Frudix, Frusemide,
Frusedale, Flusapex, Salix, Puresis, Libeo)
• torasemide
(Isemid, Torsemide, Demadex, Tormis, UpCard)
• bumetanide
* Loop diuretics are pyridine-3-sulfonurea drugs that act on the thick portion of the nephron's ascending loop of Henle (the part of a kidney tubule that forms a long loop in the medulla of the kidney, from which water and salts are resorbed into the blood), where they inhibit the sodium-potassium-chloride (Na+-K+-2Cl-) cotransporter, leaving sodium (and other ions) to be lost, with water, in the urine.
The ACVIM's Consensus Statement's recommended starting dosage of
furosemide is 2mg/kg (1 kg = 2.2 lbs)
 every 12 hours.
every 12 hours.
In an April 2018 report, a team of researchers at Tufts University's Cummings vet school examined the records of 54 MVD-affected dogs (including 9 cavaliers) in "advanced heart failure" (AHF). Their definition of AHF was the recurrence of heart failure signs despite treatment with initial doses of pimobendan, ACE-inhibitor, and furosemide. At the diagnosis of AHF, they found that for most of the 54 dogs, doses of pimobendan, furosemide, ACE-inhibitor, and spironolactone were increased, with new medications added in most dogs. They found that the median survival time after diagnosis of AHF was 281 days (range, 3-885 days), and that dogs receiving a furosemide dose >6.70 mg/kg/day had significantly longer median survival times (402 days [range, 3-885 days] versus 129 days [range 9-853 days]). They concluded that dogs with AHF can have relatively long survival times, and that "a higher furosemide dose was significantly associated with longer survival time."
Loop diuretics, including furosemide, can severely affect the kidneys by activating the renin-angiotensin aldosterone system (RAAS)*, since reduction in the total circulating blood volume results in activation of RAAS. By eliminating fluids in the dog's body, diuretics also cause a reduction in essential minerals which form the electrolytes in the body's fluids. These minerals include sodium, potassium, chloride, calcium, magnesium, phosphate, and bicarbonate. Excessive loss of these elctrolyte ninerals also cause activation of the RAAS.
Loop diuretics also can adversely affect the liver and other bodily functions, and so a baseline of the kidneys and liver should be evaluated before starting furosemide and should be monitored three to five days later (since adverse reactions to the drug typically occur within three to five days of therapy initiation) and every three months thereafter. See this December 2016 article for details about administering loop diuretics to dogs with MVD.
* When the RAAS is activated, it causes the kidneys to over-work by retaining more water and sodium and excreting more potassium. As a result of this process, the overall volume of blood increases, meaning that more blood is pumped through narrowed arteries, which also increases the blood pressure.
In a 2009 study report which did not include CKCSs, veterinary cardiologists observed a three-fold increase in RAAS activity using furosemide. Their conclusion was that "furosemide is not recommended for chronic use in the absence of concurrent therapy to blunt RAAS activity, such as ACE-I, aldosterone receptor blockers, or angiotensin II type I receptor blockers." A subsequent similar study in 2011 concluded:
"These results in clinically normal dogs suggested that furosemide, administered with or without pimobendan, should be accompanied by RAAS-suppressive treatment."
In a July 2015 study, the researchers determined that doses lower than those identified to activate the circulating RAAS were not effective in treating the disorder.
 Torasemide (Isemid, Torsemide,
Demadex, Tormis, UpCard), another loop diuretic, is more effective than
furosemide due to its relatively consistent absorption and longer
half-life. This typically results in torsemide having a longer duration
and slower urinary excretion rate than does furosemide. Torasemide often is substituted for furosemide once
furosemide seems to be losing its effectiveness (furosemide resistance)
in Stage D.
As an example, some cardiologists substitute torasemide once the
patients need more than 8 mg/kg/day of furosemide.
Torasemide (Isemid, Torsemide,
Demadex, Tormis, UpCard), another loop diuretic, is more effective than
furosemide due to its relatively consistent absorption and longer
half-life. This typically results in torsemide having a longer duration
and slower urinary excretion rate than does furosemide. Torasemide often is substituted for furosemide once
furosemide seems to be losing its effectiveness (furosemide resistance)
in Stage D.
As an example, some cardiologists substitute torasemide once the
patients need more than 8 mg/kg/day of furosemide.
The ACVIM's Consensus Statement's recommended starting dosage of torasemide is 0.1 to 0.2 mg/kg, by mouth every 12 to 24 hours. That would be approximately 5%-10% of the current furosemide dosage to deliver a furosemide-equivalent dose.
Side effects due to torasemide include dehydration, lethargy, weakness, anorexia, azotemia*, electrolyte imbalances, and abnormal ranges for (blood urea nitrogen (BUN), creatinine, sodium, potassium, chloride, and blood pressure.
* Azotemia is an abnormality consisting of abnormally high levels of urea, creatinine, and other nitrogen-rich compounds in the dog's blood.
In a 2012 report, researchers compared doses of torasemide and furosemide in treating dogs with stable congestive heart failure (Stage C). They found that "torsemide is equivalent to furosemide at controlling clinical signs of CHF in dogs and is likely to achieve greater diuresis vs. furosemide." Torasemide is approximately 10- to 20-times as potent as furosemide in dogs, depending upon the frequency of the dosages -- 10-times stronger if given once daily and 20-times if given twice. (See this October 2015 article and this November 2019 article.) Therefore, the starting dosage is approximately 1/20 that of the furosemide dose. A possible side effect of torasemide is syncope. It also only requires a single dose per day for most dogs in Stage C.
In a November 2019 article, Colorado State Univ. researchers tested 6 healthy Beagles to compare the effects of the diuretics torsemide and furosemide upon activation of the kidney renin-angiotensin-aldosterone system (RAAS). Chronic RAAS activation is known to promote sodium and water retention, vasoconstriction, heart enlargement, and renal remodeling. The goal of the study was to compare the magnitude of RAAS activation between these two loop diuretics, administered at approximately equipotent dosages. Since torsemide has been found to be 20 times more potent than furosemide, the equipotent dosage of torsemide was 1/20th that of furosemide. The researchers report finding that, over a 10-day period, the diuretics behaved similarly, including the extent of increase in RAAS activity. There also was no significant difference in the extent of potassium excretion, although furosemide caused less than did torsemide. They also confirmed that torsemide given twice daily had a potency factor close to 20 times that of furosemide.
In an October 2017 article on the TEST Study, researchers sponsored by UpCard's manufacturer found that over a short-term of 84 to 91 days, torasemide resulted in a two-fold reduction over furosemide in the risk of reaching the cardiac endpoint (either a cardiac-associated death or worsening of the degree of CHF). They concluded by warning:
"However, given its potent diuretic effects, the lowest effective dosage should always be determined and, as recommended by the ACVIM consensus guidelines, dogs under such diuretic treatment should be closely monitored for renal and electrolyte abnormalities. Further studies are now required to explore the potential beneficial antifibrotic effect of torasemide on dogs with CHF, and its potential benefit over furosemide on long-term survival."
In an August 2020 article, a team of employees of Ceva Sante Animale, manufacturer of torasemide, along with PennVet cardiologist Mark A. Oyama, compared the diuretic to furosemide in a study of 319 MVD-affected dogs diagnosed with new onset congestive heart failure (CHF), including 52 (16.3%) cavalier King Charles spaniels. Their study, titled CARPODIEM, had the aim to evaluate the efficacy and safety of torasemide compared to furosemide in treating CHF. They report finding that torasemide was superior to furosemide in that it required only once-daily dosing, resulting in increased owner compliance, and also that torasemide had less than half the risk of cardiac death or euthanasia or worsening of CHF than did furosemide. They emphasized that monitoring of the dogs' reactions, hydration status, renal function, and serum electrolyte concentrations should be performed when using any diuretic.
In a May 2023 article, a team of Iranian researchers and one from Australia compared the effects of the loop diuretics furosemide and torsemide on certain echocardiographic measurements and blood pressure, in five healthy crossbreed dogs. They found that torsemide significantly reduced blood pressure an hour after administration, while furosemide did not, and also that torsemide increased heart rate above that of furosemide. They report finding no other significant differences between the treatment groups.
See also this November 2017 article on the efficacy of long-term use of torasemide.
Bumetanide, another loop diuretic, is 25 to 50 times more potent than furosemide. Otherwise, it performs in the same manner as furosemide. Proper dosages for treating pulmonary edema (fluid in the lungs) of dogs have not been published.
Medications approved to treat humans with congestive heart failure, such as the aquaretic (vasopressin receptor anatagonist = vaptans), (tolvaptan), are being empirically considered as alternatives to diuretics such as furosemide. Vaptans block the action of vasopressin, causing removal of excess water without the loss of electrolytes. Dosages on MVD-affected dogs have been in the range of 2 to 3 mg/kg orally every 12 hous. See this November 2011 article.
RETURN TO TOP
Spironolactone:
Spironolactone is a synthetic 17-lactone drug, a mild, potassium-sparing diuretic. However, there are no published studies of the efficacy of spironolactone as a diuretic in dogs in CHF.* Therefore, it should not be substituted for furosemide or any other diruetic in dogs in CHF. It will not sustain control of the fluid retention in CHF. Spironolactone is no more sparing on the kidneys than furosemide, except to the extent that it is a less effective diuretic than furosemide.
* See this May 2016 review of the use of spironolactone as a diuretic.
There is a lot of conflicting research information about Spironolactone (Aldactone, Prilactone, CaroSpir).
While it is known as a potassium-sparing diuretic, it is not needed as such since most dogs receiving furosemide do not become potassium-depleted, especially if the furosemide is coupled with an ACE-inhibitor or aldosterone receptor blocker (AFB). In a 2010 European study, sponsored by the manufacturer of Prilactone, the investigators reported that spironolactone, when added to conventional cardiac therapy (such as an ACE-inhibitor, plus furosemide and digoxin if needed ) was found to decrease the risk of reaching the primary endpoint (ie, cardiac-related death, euthanasia, or severe worsening) in dogs with moderate to severe mitral regurgitation caused by MVD. However, in a 2010 Letter to Editor, Drs. Mark Kittleson and John Bonagura disagreed with the authors of the 2010 European study's conclusion that a survival benefit of spironolactone in canine MR was proven, stating that there was confusion as to "which dogs if any did benefit from spironolactone" along with other criticisms, including the extent of sponsor control over the study. *
*See also a 2011 report indicating that spironolactone did not extend survival times of dogs with advanced heart failure.
See mineralocorticoid receptor antagonists (below) for more information about using spironolactone in the treatment of Stage C MVD-affected dogs.
RETURN TO TOP
Thiazide diuretics (hydrochlorothiazide):
Thiazide diuretics inlude:
• hydrochlorothiazide (Dyazide, Thiuretic, Esidrix, Hydrodiuril, Microzide)
• co-amilozide
or amiloride / hydrochlorothiazide (Moduretic, Moduret), a
combination of amiloride and hydrochlorothiazide.
A thiazide diuretic is typically administered in combination with a loop diuretic (e.g., furosemide) if the loop diuretic alone does not adequately control fluid retention. When added to the loop diuretic, dosages of hydrochlorothiazide (HTCZ) range from an initial dose of 0.5 mg/kg orally every 24 hours to 2.0 mg/kg every 12 hours. Standard blood serum levels and blood pressure need to be checked periodically. Possible side effects include dehydration, azotemia, electrolyte imbalances, lethargy, weakness, and anorexia.
In a July 2021 article, a team of Japanese cardiologists studied the effects of adding hydrochlorothiazide (HTCZ) in treating 14 dogs in heart failure due to mitral valve disease (MVD) which no longer were responding to treatment with high doses of the loop diuretic torsemide. One of the 14 dogs was a cavalier. After dual treatment with the two diuretics, the echocardiographic data showed significant improvement. However, blood urea nitrogen and creatinine levels increased, and potassium levels decreased, indicating a decline in renal function following HTCZ administration. They concluded that their study suggested that the administration of HTCZ in combination with loop diuretics may improve cardiac function during advanced heart failure in MVD-affected dogs. However, as the combination of HTCZ and loop diuretics can deteriorate renal function, they advised that caution should be exercised prior to making recommendations regarding its use, and renal function should be monitored.
In a May 2025 article, Italian cardiology researchers examined the short-term and long-trm effects of hydrochlorothiazide in 38 dogs, including 4 (10.5%) cavaliers suffering from relapsing congestive heart failure (CHF) due to MVD. They report finding that, at a median of 7 days after adding HCTZ to the medication cocktail, creatinine, urea, and total calcium levels significantly increased, while sodium and potassium levels significantly decreased. While no dog developed severe electrolyte abnormalities, some dogs showed severe increases in creatinine and urea. After a median of 95 days, no significant echocardiographic changes developed. Episodes of CHF were more frequent before (median, one every 68 days) than after (median, one every 124 days) after startiing HCTZ.
In a September 2025 article, Italian researchers report results of a study of 25 MVD-affected dogs in Stage D (refractory congestive heart failure) treated with hydrochlorothiazide (HCTZ) as an additional diuretic. They started the HCTZ at 0.8 mg/kg every 48 hours and progressively increased the dosages to as high as 1.5 mg/kg per day. They observed azotemia (abnormally high levels of nitrogen-containing compounds in the blood) in 3 of the 25 dogs (12%). Azotemia can be due to insufficient or dysfunctional filtering of blood by the kidneys and can lead to uremia and acute kidney injury if not controlled. During the course of the study, 20 died, 18 due to MVD. The median survival time of the dogs was 268 days. Overall, they concluded that "HCTZ addition at the study dosages appeared clinically tolerated in most dogs with MMVD ACVIM stage D, with a prolonged median survival time for dogs in ACVIM Stage D."
RETURN TO TOP
Direct renin inhibitors (DRIs):
Direct renin inhibitors (DRIs) include:
• carperitide
• NPA7
• aliskiren (Tekturna, Rasilez).
Carperitide, an alpha-human atrial natriuretic peptide, is a human drug which is known to reduce pressure in the left atrial and left ventricle chambers of the human heart. In an August 2013 report, a team of Japanese veterinary heart surgeons compared dosing lab dogs with carperitide and furosemide. The team reported that both drugs similarly reduced left atrial pressure. They found that carperitide had less adverse effects than furosemide because it did not activate the renin-angiotensin-aldosterone system (RAAS). They concluded that additional studies are warranted in clinical patients with degenerative MVD and congestive heart failure.
In a June 2017 abstract, Japanese cardiologists tested MVD-affected dogs which also had pulmonary edema, using carperitide. Factors such as atrial natriuretic peptide (ANP), N-terminal pro b-type natriuretic peptide (NT-proBNP), and aldosterone levels, were measured before and after administration of the drug. In addition, chest radiography, echocardiography, and blood pressure measurements were performed depending on the specific cases. Seven of the 9 dogs showed improvements post-treatment, although 2 cases did not show any improvement. ANP levels increased post-treatment in all cases. NT-proBNP levels increased in 4 cases and decreased in 5 cases. Aldosterone level increased only in one case. In addition, the left atrial aortic root ratio (LA/Ao), E-wave, vertebral heart size (VHS), and blood pressure decreased. They observed a decrease in NT-proBNP levels in all cases that were treated for more than 7 hours. In addition, the ventricular muscle stretch was reduced by the decrease in left atrial pressure following the administration of the carperitide, which lasted for more than 7 hours. Finally, the decrease in aldosterone levels was thought to be due to the inhibitory effects of carperitide on the renin-angiotensin-aldosterone system. They concluded that carperitide administration may be effective since pulmonary edema improved and aldosterone was suppressed.
In a May 2017 abstract, Mayo Clinic researchers reported on the success of their engineered designer natriuretic peptide "NPA7" as co-therapy with diuretics (furosemide) in treating ten dogs in acute decompensated heart failure (ADHF). They noted that furosemide (FURO) alone lacks vasodilatory actions, impairs kidney function, and activatesd the renin-angiotensin-aldosterone system (RAAS). They found NPA7 to decrease vascular resistance, reduce pulmonary capillary pressure, increase renal blood flow, increase urinary flow and sodium excretion. They concluded:
"In conclusion, in experimental ADHF, NPA7 is a vasodilatory therapeutic with cardiac unloading, diuretic and natriuretic actions. FURO is more diuretic, but is associated with significant renal impairment, vasoconstriction and RAAS-activation. In addition, pre-treatment with NPA7 enhances natriuresis and diuresis while preserving kidney function. Priming with NPA7 may therefore represent a novel renoprotective strategy for treatment of ADHF. Importantly, this study underscores the need for future studies assessing the impact of timing of diuretics on treatment effects of novel therapies in patients with ADHF."
RETURN TO TOP
Natural alternative diuretics in Stage C
Natural diuretics include urea (AC Carbamide) by Standard Process, and Wu Ling San by Mayway and Alisma by Seven Forests, all being traditional Chinese herbal medicines (TCM).
Salvia Shou Wu is a proprietary combination of herbs (salvia root, polygonum, crataegus, peony (red), astragalus root, achyranthes, loranthus, tangkuei, dalbergia, licorice) by Seven Forests, which is designed to enhance blood circulation, dispense stagnant blood, and nourish the blood. Holistic veterinarians recommend this product for dogs in heart failure due to MVD, at the rate of 1/4 tablet twice daily with food, for dogs the size of cavaliers.
Wu Ling San is a proprietary blend of herbs (hoelen fungus, alisma root, polyporus, cinnamon, and atractylodes root) which is designed to promote urination and leaches out dampness. Holistic veterinarians recommend this product for dogs with enlarged hearts or in heart failure, due to MVD, at the rate of 1/4 teaspoon twice daily with food, for dogs the size of cavaliers.
Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained in TCM. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
RETURN TO TOP
--- pimobendan in Stage C
 In advanced heart failure, the heart muscle may become weakened so that
it does not contract properly. This is known as "systolic dysfunction",
which occurs when the heart muscle does not contract with enough force to
pump enough oxygen-rich blood throughout the body. It also is referred to as
"diminished contractility". During an echocardiogram, the cardiologist is
able to calculate the "ejection fraction" and the "fractional shortening"
percentage (FS%), which measure how well the heart
pumps with each beat. That ejection fraction and/or the FS% determine whether there is
systolic dysfunction.
In advanced heart failure, the heart muscle may become weakened so that
it does not contract properly. This is known as "systolic dysfunction",
which occurs when the heart muscle does not contract with enough force to
pump enough oxygen-rich blood throughout the body. It also is referred to as
"diminished contractility". During an echocardiogram, the cardiologist is
able to calculate the "ejection fraction" and the "fractional shortening"
percentage (FS%), which measure how well the heart
pumps with each beat. That ejection fraction and/or the FS% determine whether there is
systolic dysfunction.
Reducing pressure in the arteries can make it easier for the heart to pump. Pimobendan (Vetmedin, Cardisure, Safeheart, Pimocard, Pimomedin, Pimotab, Zelys) works in two separate ways. First, it relaxes and widens the blood vessels, allowing more more blood to flow from the heart in the proper direction, thereby reducing the congestion of blood in the heart and reducing the blood pressure. This is called vasodilating. Second, it strengthens the heart muscle, enabling it to pump more efficiently. This is called an inotropic effect, and it works on the heart muscle's contractility.
Pimobendan typically is prescribed initially at the dosage rate of 0.2 to 0.3 mg/kg (1 kg = 2.2 lbs) every 12 hours on an empty stomach. The Vetmedin website and its Client Information Sheet (below) recommend giving it an hour before meals. A recent meal reportedly significantly decreases absorption.

Technically speaking, pimobendan is a benzimidazole pyridazinone derivative and is classified as an inodilator drug with both an inotropic effect (meaning that it modifies the force or speed of contraction of muscles) and as an arteriolardilator (meaning that it widens blood vessels, allowing blood to flow more easily), promoting increased cardiac output, reduction both in preload and afterload and increased myocardial contractility without increases in myocardial oxygen and energy consumptions. See the "A Few Words About Pimobendan" box below for more details.
In a July 2015 opinion, the European Medicines Agency's Committee for Medicinal Products for Veterinary Use has recommended granting marketing authorization for Fortekor Plus, a combination of benazepril and pimobendan. The committee warns that the combination "should only be used in patients whose clinical signs are successfully controlled by administration of the same doses of the individual components (pimobendan and benazepril hydrochloride) given concurrently."
Pimobendan may have any of several disturbing side effects. A list of its adverse reactions, published bythe maker of its brand name, Vetmedin, is here:

Depending upon the serverity of the reaction, the cardiologist may recommend reducing the dosage or eliminating the drug.
RETURN TO TOP
--- dual therapy (diuretic and pimobendan)
Prior to the onset of congestive heart failure (lung edema -- Stage C), the only drug prescribed for cavaliers diagnosed with MVD in Stage B2 has been pimobendan. Once the MVD-affected dog progressed to Stage C, the dog continues to be treated with pimobendan, and a diuretic, usually furoseimde, is added. This is known as dual therapy (DT).
In an August 2025 article, Italian researchers compared the results of treating 211 dogs diagnosed to be in Stage C, which were treated with three combinations of medications. Thirty of the dogs were cavaliers (14%), the breed with the most dogs in the study. The combinations were (1) dual therapy -- DT (furosemide and pimobendane) 65 dogs, (2) triple therapy -- TT (furosemide, pimobendan, and benazepril) 105 dogs, and (3) quadruple therapy -- QT (furosemide, pimobendan, benazepril, and spironolactone) 41 dogs. All of the dogs were being treated at veterinary schools in Italy. The researchers report that no statistically significant differences were observed among the DT, TT, and QT. They found that the QT and TT did not provide a higher clinically evident cardiac protective effect compared to DT. They concluded that DT was not inferior to TT and QT in terms of cardiac death in dogs with Stage C MVD included in this study.
RETURN TO TOP
--- ACE-inhibitors in Stage C
ACE stands for angiotensin-converting enzyme. ACE-inhibitors (ACE-I) are intended to block the angiotensin converting enzyme, which is necessary to produce a substance that causes blood vessels to tighten. So, ACE-I serve to relax the blood vessels, thereby lowering the blood pressure and increasing the supply of blood and oxygen to the heart. However, studies have shown only a low concentration of angiotensin II receptors in dogs' affected mitral valves.
ACE-inhibitors include:
• Enalapril (Enacard, Vasotec, Prilenal, Envas)
• Benazepril (Lotensin, Fortekor, Banacep Vet, Benefortin, Benazecare, VetACE, Prilben,
Nelio)
• Imidapril (Tanatril)
• Ramipril (Altace, Tritace, Vasotop)
• Alacepril
• Captopril (Capoten)
• Fosinopril
• Lisinopril (Zestril, Prinivil,
Qbrelis)
•
Quinapril (Accupril)
• Temocapril (Acecol)
• Zofenopril
ACE-inhibitors (ACE-Is) usually also will be prescribed to dogs in Stage C. ACE inhibitors have been believed to block the angiotensin converting enzyme, which is necessary to produce a substance that causes blood vessels to tighten. So, ACE-Is serve to relax the blood vessels, thereby lowering the blood pressure and increasing the supply of blood and oxygen to the heart. The result is that in dogs in heart failure (HF), they are intended to blunt the enlargement of the heart and slow the progression of heart failure.*
* See, the IMPROVE Study, the COVE Study, and this 1997 article.
The ACVIM's Consensus Statement's recommended starting dosage of an ACE-inhibitor is 0.5mg/kg (1 kg = 2.2 lbs) every 12 hours.
Nevertheless, the most recent studies of ACE-I, especially the VALVE Study, have concluded that ACE-Is serve no useful purpose in treating MVD, even at Stages C and D.
The most popular ACE-inhibitors among veterinary cardiologists are enalapril and benazepril. There are a few differences between enalapril and benazepril. For example, enalapril is excreted fully through the kidneys, while benazepril is excreted 50% by the kidneys and 50% by the liver, Therefore, for dogs diagnosed with kidney problems, benazepril likely will be prescribed instead of enalapril. Because of the possible affect of any ACE-inhibitor upon the kidneys, the best practice is for the dog's renal system to be re-examined within a week to ten days following starting being given the drug.
A few studies have concluded that diuretics such as furosemide should be used only combined with ACE inhibitors -- which also prevent fluid retention -- so that the diuretic dosage may be sharply reduced to avoid the worst of its negative side effects. Researchers have found that extensive use of diuretics alone may contribute to renal dysfunction by activating the renin-angiotensin aldosterone system (RAAS) , as well as dehydration, azotemia (elevation of blood urea nitrogen [BUN]), and hypokalemia (low potassium).
However, in a September 2014 study of ten healthy hounds, researchers found that benazepril did not prevent the activation of the renin-angiotensin aldosterone system (RAAS), a cause of renal dysfunction. This is called "aldosterone breakthrough" (ABT). In a similar study in September 2015, researchers found that enalapril also did not prevent activation the RAAS. In a November 2015 study, researchers found that high doses of benazepril or enalapril were not any more effective than standard doses of those drugs in suppressing the RAAS. In a May 2017 report, the investigators found that ABT occurs in approximately 30% of MVD-affected dogs receiving ACE-inhibitors.
A baseline renal panel should be conducted before treatment is initiated and again three to five days later. Adverse reactions to the drug typically occur within three to five days of therapy initiation.
In a July 2015 opinion, the European Medicines Agency's Committee for Medicinal Products for Veterinary Use has recommended granting marketing authorization for Fortekor Plus, a combination of benazepril and pimobendan. The committee warns that the combination "should only be used in patients whose clinical signs are successfully controlled by administration of the same doses of the individual components (pimobendan and benazepril hydrochloride) given concurrently." In a July 2017 article, a team of investigators employed by Elanco Animal Health, which markets Fortekor Plus, conducted a study of 67 dogs (including 9 cavalier King Charles spaniels) at a clinic in Japan. All of the dogs were in congestive heart failure (Stage C) due to mitral valve disease (MVD). The dogs were divided into three groups. The test group received the Fortekor Plus, while two control groups received separate doses of benazepril and pimobendan. The researchers found no significant differences between groups. They reported that the frequency of vomiting (emesis) was significantly lower in the Fortekor Plus group. They concluded that:
"Fortekor Plus had non-inferior efficacy and was associated with significantly less emesis compared to administration of Fortekor and Vetmedin in dogs with CHF caused by MMVD."
In a September 2020 article -- The VALVE Study, a team of German and Swiss researchers compared treating MVD-affected dogs in congestive heart failure (CHF - Stage C) with and without the ACE-Inhibitor ramipril. They divided 158 dogs (only 8 cavaliers - 5%) diagnosed with MVD and in CHF into two groups. Group DT (double therapy) consisted of 77 dogs (including 4 cavaliers - 5%) treated with furosemide (Lasix) and pimobendan (Vetmedin). Group TT (triple therapy) included 79 dogs (4 CKCSs - 5%) treated also with ramipril. They hypothesized that the addition of the ACE-Inhibitor would extend the survival time compared to the double therapy group. They found and concluded:
"In conclusion, the addition of an ACEI [ACE-Inhibitor] to pimobendan and a diuretic, as recommended in the ACVIM guidelines for the treatment of chronic stage C MMVD, did not have a significant effect on survival time in dogs with CHF caused by MMVD. Therefore, recommending TT as standard treatment for such dogs does not seem justified."
Indeed, the results of this study showed that not including ramipril extended the average survival time by 41 days longer than adding the ACE-Inhibitor to the treatment cocktail. In effect, ramipril caused the MVD to progress more rapidly than just treating with a diuretic and pimobendan.
In a March 2018 article, a team of Japanese cardiologists switched 73 MVD-affected dogs in CHF, including 6 cavalier King Charles spaniels, from other ACE-inhibitors (either benazepril, enalapril, ramipril, or temocapril), to the ACE-inhibitor alacepril. All dogs had been treated with the other ACE-inhibitors for at least one month prior to the switch. They compared the degrees of mitral valve regurgitation (HR), activeness, appetite, responsiveness, body weight, and cough frequency and found "significant improvement" following two weeks of treatment with alacepril. After four weeks of alacepril treatment, they report observing an overall reduction of murmur intensity. They concluded:
"In this study, switching to alacepril from other ACE-Is has shown to reduce HR, and improve the clinical symptoms of CHF, in particular the frequency of cough. The improvement of the clinical symptoms was related to the improvement in the class of heart failure. In addition to the influence on the renin-angiotensin-aldosterone system (RAAS), alacepril is known to have a wide range of actions, including inhibitory effects on the sympathetic nervous system and negative chronotropic effects. Although with the results of this study one cannot be certain, however, it is most likely that multiple factors are involved in the cough suppressive effect of alacepril."
In an October 2017 article, researchers reported that a polymorphism* of an ACE gene, intron 16, is most commonly found in the CKCS, and that MVD-affected dogs with that mutation tend to have significantly lower ACE activity. In a December 2017 article, the same team of researchers studied 73 cavalier King Charles spaniels and reported finding that:
"The CKCS appears to have a high prevalence of the ACE variant. Dogs with the ACE variant had lower levels of ACE activity even in more advanced mitral valve disease than dogs without the variant."
They concluded that the impact of their finding "on the need for ACE-I in dogs with the polymorphism and heart disease deserves further study."
Then, in a September 2023 abstract, the same authors' aim was to determine the impact of certain angiotensin converting enzyme (ACE) gene polymorphisms upon the presence of congestive heart failure (CHF) in MVD-affected cavaliers. They included 182 CKCSs in their study, of which 107 were diagnosed with CHF and 75 others, all over 8 years of age, were not in CHF. They report finding that cavaliers with an ACE polymorphism were less likely to have CHF compared to CKCSs without a polymorphism. They concluded that an ACE polymorphism may play a role in protecting MVD-affected cavaliers from reaching CHF. They also included results of their study of polymorphisms of the cavaliers' phosphodiesterase system genes, and concluded that CKCSs with the PDE5A polymorphism gene had increased odds for CHF compared to dogs without that mutation.
* Polymorphism refers to the presence of two or more variant forms of a specific DNA sequence that can occur among different dogs or breeds.
In a July 2021 article, this same team identified studied the gene for the ACE enzyme -- canine chromosome 9:11507816:G > A -- in 497 dogs of 54 breeds, including 13 cavaliers, to determine the prevalance of a variant of that gene which prevents ACE-inhibitors from suppressing the RAAS and instead allows aldosterone breakthrough. The ACE gene variant was found in 32 of the 54 breeds. The variant was greatest five breeds, including the CKCS with a prevalence of 85%. The other four breeds were the Irish wolfhound (95%), Dachshunds (90%), great Danes (84%), and bull mastiffs (58%).
In a February 2020 article, Dr. Mark Rishniw carefully dissected all five drug-company-funded studies of the ACE-inhibitor enalapril (the IMPROVE, COVE, LIVE, SVEP, and VETPROOF studies) to determine whether the drug would delay the onset of cardiac death in MVD-affected dogs in heart failure (CHF) or would delay the onset of CHF in pre-clinical MVD-affected dogs (i.e., in Stages B1 or B2). While the conclusions of some of the studies were muddled by drug-company interference, Dr. Rishniw's analysis of the underlying data showed that ACE-inhibitors have not been scientifically shown to delay the onset of either CHF or cardiac death due to MVD. He goes on to determine that:
"In retrospect, it became clear that the veterinary community was hoodwinked into believing that ACE-I were the medical cure for CHF dogs because of corporate marketing decisions."
"So, what does all this mean? My interpretation of all this literature suggests that ACE-I have no place in managing mitral valve disease, whether in dogs with CHF or dogs with subclinical disease."
RETURN TO TOP
--- natural alternatives to ACE-inhibitors in Stage C
A natural supplement as an alternative to ACE-inhibitors is a combination of active fish petides, including LKPNM, from the bonito fish (Sarda orientalis), such as Vasotensin, manufactured by Metagenics, Inc., and PeptACE by Natural Factors. Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained in TCM. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
Other Chinese herbal alternatives include Salvia Shou Wu, a Seven Forests patented supplement which consists of Salvia extract, and several other herbs and flowers. Salvia Shou Wu encourages blood circulation.
LASSBio 897 is a new prototype drug produced from safrole substrate, a compound extracted from the "sassafras oil", found in Brazilian plants like "canela-branca" (Ocotea pretiosa), caused vasodilation after two hours of their administration, similar to what was observed with benazepril.
RETURN TO TOP
--- triple therapy (diuretic, pimobendan, ACE-inhibitor)
Triple therapy (TT) refers to adding an ACE-inhibior to the double therapy (DT) of pimobenan and furosemide to the MVD-affected cavalier in Stage C. This is notwithstanding the VALVE Study and the polymorphism discussed above.
In an August 2025 article, Italian researchers compared the results of treating 211 dogs diagnosed to be in Stage C, which were treated with three combinations of medications. Thirty of the dogs were cavaliers (14%), the breed with the most dogs in the study. The combinations were (1) dual therapy -- DT (furosemide and pimobendane) 65 dogs, (2) triple therapy -- TT (furosemide, pimobendan, and benazepril) 105 dogs, and (3) quadruple therapy -- QT (furosemide, pimobendan, benazepril, and spironolactone) 41 dogs. All of the dogs were being treated at veterinary schools in Italy. The researchers report that no statistically significant differences were observed among the DT, TT, and QT. They found that the QT and TT did not provide a higher clinically evident cardiac protective effect compared to DT. They concluded that DT was not inferior to TT and QT in terms of cardiac death in dogs with Stage C MVD included in this study.
RETURN TO TOP
--- Spironolactone & mineralocorticoid receptor antagonists (MRA)
Mineralocorticoid receptor antagonists (MRA), also referred to as aldosterone receptor antagonists, include:
• spironolactone
• eplerenone (Inspra)
• finerenone
• Losartan potassium (Cozaar)
Aldosterone is a hormone in the mineralocorticoids group. Its function is to regulate blood pressure and sodium potassium levels. High levels of this hormone may have harmful effects in the heart, including as mechanism of cardiac remodeling, meaning enlargement.
Spironolactone has been approved within the European Union for use in
dogs with clinical signs of HF secondary to MVD, as additional therapy. It
typically acts as a comparatively weak diuretic, when
administered alone, but has synergistic effects when combined with other
diuretics. The main reason for prescribing it is to protect the dog against
the harmful effects of aldosterone on the heart and blood vessels.
However, spironolactone reportedly may lead to excessively high, life-threatening levels of potassium in the dog's blood, particularly when combined with ACE inhibitors. Some veterinary cardiologists recommend that potassium levels be carefully monitored when using spironolactone in combination with ACE inhibitors by drawing blood at regular intervals until it is evident that the potassium level is or is not going to be a problem.
The usual dosage of spironolactone is 1 to 2 mg/kg once a day with food.
In a 2013 study of the possible increased risk of adverse events for dogs taking spironolactone in addition to conventional therapies, the researchers concluded that dogs with heart failure receiving spironolactone in addition to conventional treatment are not at a higher risk for any adverse events, death caused by cardiac disease, renal disease, or both, hyperkalemia, or azotemia. The study was funded by the manufacturer of Prilactone. See also this May 2016 review of the use of spironolactone as a diuretic.
In a May 2021 article, Dr. Clarke Atkins reported the results of a study (the "BESST Study") of 569 dogs (including 40 cavaliers -- 9.66% -- the highest number of a single breed) in a comparison of treating MVD-affected dogs in Stage C (heart failure) with the ACE-inhibitor benazepril (BNZ) alone or adding spironolactone (S+BNZ). In both groups of dogs -- the BNZ group and the S+BNZ group -- the loop diuretic furosemide also was administerered. Twenty cavaliers were in each of the two groups. Of the 569 dogs, 155 were excluded for various reasons, leaving only 414 to participate in the efficacy portion of the study. Twenty-seven specialty clinics in the USA participated in the study. Spironolactone is a mineralocorticoid receptor antagonist (MRA) intended to inhibit the dogs' renin-angiotensin-aldosterone system (RAAS) from activiating in response to furosemide's effects upon the kidneys. ACE-inhibitors also are designed to inhibit activation of the RAAS system. See this section for details about ACE-inhibitors in general. The authors concluded:
"A combination of the MRA, spironolactone, and the ACE-I, benazepril, in a fixed 8:1 ratio, is more effective than benazepril alone when included in the medical management of new-onset CHF, due to MMVD. The use of the combination of spironolactone and benazepril results in reduced or delayed recurrence of CHF and its associated clinical signs, while reducing death rate over the 12-month study. Furthermore, under clinical field conditions, the safety profile of the combination product is indistinguishable from that of benazepril alone."
Cardalis is a tablet which combines spironolactone with the ACE-inhibitor benazepril. A proglem with Cardalis is that its proportions of spironolactone and benazepril are fixed, so that if the cardiologist wants to increase one without increasing the other, the Cardalis tablet will not permit it. Therefore, many cardiologists prefer to presecribe spironolactone and benazepril separately rather than as a single Cardalis dosage.
Aldactazide is a combination of spironolactone and hydrochlorothiazide (see thiazide diuretics, below), which inhibits the activity of aldosterone. Aldactazide is intended to block the adverse effects of aldosterone on the heart muscle and enhance the diuretic effects.
Other aldosterone receptor blockers (ARBs) include eplerenone (Inspra), finerenone, and Losartan potassium (Cozaar).
RETURN TO TOP
--- quadruple therapy (diuretic, pimobendan, ACE-inhibitor, spironlactone)
Quadruple therapy (QT) refers to adding spironolactone to the triple therapy (TT) of pimobenan, furosemide, and an ACE-inhibior to the MVD-affected cavalier in Stage C. This is notwithstanding the VALVE Study and the polymorphism discussed above.
In an August 2025 article, Italian researchers compared the results of treating 211 dogs diagnosed to be in Stage C, which were treated with three combinations of medications. Thirty of the dogs were cavaliers (14%), the breed with the most dogs in the study. The combinations were (1) dual therapy -- DT (furosemide and pimobendane) 65 dogs, (2) triple therapy -- TT (furosemide, pimobendan, and benazepril) 105 dogs, and (3) quadruple therapy -- QT (furosemide, pimobendan, benazepril, and spironolactone) 41 dogs. All of the dogs were being treated at veterinary schools in Italy. The researchers report that no statistically significant differences were observed among the DT, TT, and QT. They found that the QT and TT did not provide a higher clinically evident cardiac protective effect compared to DT. They concluded that DT was not inferior to TT and QT in terms of cardiac death in dogs with Stage C MVD included in this study.
RETURN TO TOP
--- combinations in one pill
Some versions of the foregoing Stage C drugs (or other MVD drugs) are being offered in combination forms. An example is Cardalis, which is a tablet which combines the ACE-inhibitor benazepril with spironolactone. Aldactazide is a combination of spironolactone and hydrochlorothiazide, which inhibits the activity of the hormone aldosterone. A downside of such combinations is that when the dosages are fixed in a combination form of more than a single drug, the cardiologist loses the options of varying the doses of each drug for each patient.
A particularly ambitious combination is ForteGold, which combines torsemide (0.2 mg), pimobendan (0.6 mg), enalapril maleate(1.0 mg), and spironolactone (2.0 mg) in one tablet. It is dosed at the rate of one tablet per 2 kg. of body weight, every 12 hours.
In an August 2025 article, Korean researchers (Jiyoung Park, Jiyoon Lee, Sang-Joon Lee, Changbaig Hyun [right]) studied the effectiveness of a tablet (ForteGold) which combined one pill a fixed dose of four drugs typically prescribed for dogs in Stage C of mitral valve disease (MVD): torsemide (0.2mg), pimobendan (0.6mg), enalapril maleate (1.0mg), and spironolactone (2.0mg), dosed at one tablet per 2kg of body weight, administered twice daily, over 56 days, compared with single doses of the same drugs in a compounded powder form, to 30 dogs in each group. Torsemide is a diuetic which rarely is prescribed initially and instead is switched for furosemide if and when furosemide begins to fail being effective. Apart from that, this combination of medications is a standard tratement, often called an MVD cocktail or "quad therapy". The authors report that:
"Both groups exhibited significant improvements in clinical signs, including exercise intolerance, appetite, respiratory effort, and coughing, with the ForteGold group showing earlier and more sustained responses. Blood chemistry and electrolyte levels remained within normal ranges, indicating a favorable safety profile. Radiographic assessments revealed a gradual decrease in vertebral heart score and normalization of lung fields by Day 56 in both groups. Echocardiographic parameters (LA/Ao, LVIDd/Ao, MVE) improved significantly, with the ForteGold group demonstrating earlier enhancements. NT-proBNP levels decreased in both groups, with a more pronounced reduction in the ForteGold group. No significant changes were observed in SDMA and cPL levels, suggesting stable renal and pancreatic functions."
They concluded that "The fixed-dose combination tablet (Sentorphil® ForteGold) offers a clinically effective and safer alternative to compounded powdered medications for managing MMVD in dogs. Its formulation ensures accurate dosing, improved owner compliance, and enhanced clinical outcomes."
The main drawbacks to ForteGold are: (1) rarely are Stage C dogs started out with toresemide as the prescribed diuretic. Maybe in Korea, but not in the rest of the world, the go-to first choice of a duretic is furosemide. And (2) when the dosages are so limited in a combination form of four drugs, the cardiologist loses the options of varying the doses of each drug for each patient.
The researchers did not compare their multi-tablet with the brand-name drugs produced by their original patent holders. The "control group" of 30 dogs was given "compounded powdered" versions of those drugs, presumably prepared by an independent pharmacy, and not the brand-name torsemide tablet, or the Boehringer Ingelheim Vetmedin tablet, and so on. As noted by the authors in their article, "compounded powdered mixtures, which may lead to dosing inaccuracies and reduced compliance." As they stated specifically:
This study may have relevance only to Korea and other limited territories in Asia. But as for the rest of us. if we can obtain brand-name pills for each of the four ingredients, the flexibility that various dosages offer should trump this fixed-dosage combination tablet.
RETURN TO TOP
--- Sacubitril/valsartan (Entresto)
 Sacubitril/valsartan
(Entresto, Sacufox V, Vymada, LCZ696) is a combination of valsartan, an
angiotensin
II receptor blocker
(ARB), which prevents vasoconstriction thereby lowering blood pressure
and improving blood flow. and sacubitril, a neutral endopeptidase (neprilysin -- NEP)
blocker, that prevents the breakdown of natriuretic peptides, which
helps lower blood pressure by reducing sodium levels. It is intended to promote diuresis and natriuresis and
counteract the effects of the kidneys'
renin-angiotensin-aldosterone system (RAAS) once the RAAS is
activated. Entresto's primary medical purpose is to suppress the RAAS.
Thus, it is being used as an alternative to
ACE-inhibitors in Stage C and D MVD-affected dogs. A dosage ranges from
5 to 10 mg/kg to 20 mg/kg orally every 12 hours.
Sacubitril/valsartan
(Entresto, Sacufox V, Vymada, LCZ696) is a combination of valsartan, an
angiotensin
II receptor blocker
(ARB), which prevents vasoconstriction thereby lowering blood pressure
and improving blood flow. and sacubitril, a neutral endopeptidase (neprilysin -- NEP)
blocker, that prevents the breakdown of natriuretic peptides, which
helps lower blood pressure by reducing sodium levels. It is intended to promote diuresis and natriuresis and
counteract the effects of the kidneys'
renin-angiotensin-aldosterone system (RAAS) once the RAAS is
activated. Entresto's primary medical purpose is to suppress the RAAS.
Thus, it is being used as an alternative to
ACE-inhibitors in Stage C and D MVD-affected dogs. A dosage ranges from
5 to 10 mg/kg to 20 mg/kg orally every 12 hours.
In an April 2014 poster presentation before the American College of Cardiology, Dr. Jonathan Mochel and others reported on their comparison cross-over study of LCZ696 (Entresto), valsartan alone, benazepril, and placebo on 18 RAAS-affected beagles for 10 days. The dosages of LCZ696 were 15 and 45 mg/kg. The aim was to determine reduction in aldosterone exposure. They found that, compared to placebo, benazepril modestly reduced AL, and valsartan and LCZ696 decreased AL levels to a significant extent (-23%, −45% and −43%, respectively, p<0.05). The greatest reductions were observed in the LCZ696 groups, with LCZ696 which at 2 hours reduced AL 2-fold lower than valsartan.
In an August 2018 article, a team of Auburn University researchers examined the effectiveness of sacubitril/valsartan (S/V) in 7 dogs diagnosed with mitral valve disease (MVD), compared to 6 MVD-affected dogs in the placebo group. They report finding that S/V is effective at lowering the urine concentration of aldosterone and that it was safe, with no deleterious effects on BUN, creatinine, and electrolyte concentrations, or systolic arterial pressure.
In a February 2019 article, the investigators compared the effects of sacubitril/valsartan versus just valsartan, and versus benazepril, on the dynamics of the renin-angiotensin-aldosterone system (RAAS) and the natriuretic peptides (NP) system in 18 laboratory beagle dogs after activating their RAAS with a low-salt diet over 15 days. They reported:
"In conclusion, the ARNI sacubitril/valsartan reduced ALD, a known risk factor of CV mortality, and enhanced the NP system via cGMPmediated pathways in a low-sodium diet model of RAAS activation. The results presented herein provide further evidence that the effects on the renin cascade extend to reduced ALD levels beyond that achieved with RAAS blockade alone. These positive findings in dogs also suggest that sacubitril/valsartan is a promising pharmacological candidate for increased survival in canine cardiovascular diseases."
In a November 2020 article, 7 dogs in which cardiorenal syndrome (CRS) was induced, resulting in heart failure and renal dysfunction, were treated with S/V for 3 months and compared to 7 other dogs in a control group. The investigators reported that the S/V improved left ventricle systolic function and mitochondrial function and decreased biomakers of heart and kidney injury.
In a July 2021 article, Thai investigators compared sacubitril/valsartan with the ACE-inhibitor ramipril in treating dogs diagnosed with Stage C (heart failure) due to MVD, along with both pimobendan and the diuretic furosemide. Twenty-one dogs -- none being cavaliers -- were divided into the S/V group (11 dogs) and the ramipril group (10 dogs) for four weeks. The investigators report that short-term administration of SV in dogs with MVD stage C resulted in a greater extent of reverse myocardial remodeling of both the left atrium and the left ventricle than in the ramipril group, as indicated by several echocardiographic parameters. They concluded:
"The current study suggested that the short-term effects of SV can reverse myocardial remodeling, as inferred from several echocardiographic indices (i.e., the reduction in LA/Ao, LVIDDN, EDVI and ESVI) in dogs with MMVD stage C. These findings would support the use of SV in clinically symptomatic heart failure in dogs."
However, they warn of a few limitations: (a) a very small sample size, with a much larger sample size needed to confirm the findings; (b) the study was for a very short term -- four weeks -- and a longer term study should be conducted; (c) the results thus far "do not suggest that ACEi should be replaced with S/V in dogs with MMVD stage C."
In a June 2024 article, Japanese resaerchers studied the effects of Entresto on the kidneys of 5 healthy laboratory dogs. They were given oral doses of 20 mg/kg. twice a day for 4 weeks. Kidney blood flow (renal hemocynamics) was assessed before the first dose and then on days 7 and 28. They report finding that Entresto "may enhance renal haemodynamics in healthy dogs." Translated, this means Entresto may improve the blood flow through the kidneys.
At the June 2025 ACVIM Forum, Dr. Justin Carlson presented a lecture, "Beyond the Guidelines: Sacubitril/Valsartan (ARNi) (Entresto)", in which he reported on 49 case studies of MVD-affected dogs in late Stage C or Stage D ("pretty bad cases that I thought were not going to do very well"), 7 of which were cavalier King Charles spaniels (and 7 Maltese and 6 Yorkies). Among those 49 patients in which he substituted Entresto (sacubitril/valsartan - S/V) for an ACE-inhibitor (such as benazepril or enalapril) and spironolactone, their mean survival time was 474 days, with a median of 381 days, and an overall survival range from 152 to 1,989 days. He compared those results to those of the VALVE Study trial and the QUEST Study. In the VALVE Study of Stage C dogs (in which the ACE-inhibitor ramipril was added to the combination of furosemide and pimobendan), the median survival time was only 214 days. In the QUEST Study (in which Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan alone. He emphasized that the patients in his group of 49 dogs being treated with Entresto were late Stage C or Stage D dogs, meaning that they were in far worse shape than the Stage C dogs just developing heart failure which were the subjects of the VALVE and QUEST studies.
Dr. Carlson said that the owners of 39 of his 49 patients (80%)
reported their dogs had noticeably improved
 energy levels. The owners of
28 of the dogs (57%) reported that their dogs were coughing less. He
found that "in a lot of these dogs", the S/V allowed for the safe
reduction of diuretics, and in "a handful of these dogs", he has since
stopped any diuretic medications, and "they are doing great." He
reported that, over time, there was "a statistically significant
difference" in the sizes of the left ventricle and left atrial ratio. He
found in some dogs that over time their left atriums were back to normal
in size.
energy levels. The owners of
28 of the dogs (57%) reported that their dogs were coughing less. He
found that "in a lot of these dogs", the S/V allowed for the safe
reduction of diuretics, and in "a handful of these dogs", he has since
stopped any diuretic medications, and "they are doing great." He
reported that, over time, there was "a statistically significant
difference" in the sizes of the left ventricle and left atrial ratio. He
found in some dogs that over time their left atriums were back to normal
in size.
As for adverse effects, he had only 2 of the 49 dogs with gastrointestinal distress. As for renal values, only one patient had significant worsening renal values, with 3 having increased azotemia in 4 months and 5 having significantly decreased renal values after 4 months. Regarding survival times, the mean survival time was 474 days, with a median of 381 days , and a range from 152 to 1,989 days. He pointed out that in the VALVE Study trial, in which an ACE-inhibitor (ramipril) was added to the combination of furosemide and pimobendan, the median survival time was only 214 days, and in the QUEST Study (in which the Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan. He emphasized that the patients in his group of dogs being treated with Entresto were Stage D dogs.
Dr. Carlson recommends "up-titration" of dosages of S/V, meaning starting at a low dosage and then increasing it, over 2 to 4 weeks, depending upon the dog's response and the blood test results. He stops both ACE-inhibitors and spironolactone when he starts the S/V, waiting 36 hours. His recommended dosage range in 15-30 mg/kg twice a day.
In a paper presented at the 2025 ACVIM Forum, cardiologists Ryan Fries, Elizabeth Malcolm, Sonya Gordon, and Justin Carlson wrote:
"When utilizing Entresto in a group of canine patients (n=49) with congestive heart failure secondary to myxomatous mitral valvular disease or dilated cardiomyopathy, Entresto was well tolerated, with fewer than 5% of patients having undesirable side effects when titrated appropriately. While a FETCH questionnaire was not completed on patients, there was an overwhelming owner-noted improvement to the patients' quality of life when optimal dosing of Entresto was achieved based on owner report history during follow-up. Up-titration of the medication was done over the span of 1-6 weeks in most patients, with some patients requiring an extended up-titration over multiple months. Survival, echocardiographic, and dose-response data will be presented in a future manuscript. Based on the encouraging data from this cohort of patients, the optimal dosing, timing of up-titration, and ideal start time in patient disease stage warrant further study in a prospective manner."
The U.S. Food & Drug Administration (FDA) approved S/V for humans in 2015 to reduce the risk of cardiac death in patients with chronic heart failure. Valsartan (Diovan) alone had been approved by the FDA in 2002 for treating humans of heart failure. FDA approval of S/V for humans was based upon a study (PARADIGM-HF) comparing it to enalapril in 8,442 patients with symptomatic chronic heart failure and systolic dysfunction. Studies of humans recommend up-titration from the lowest dose to the highest tolerable dose over periods of 2 to 4 weeks. It is for these reasons that veterinary cardiologists in general prefer to wait until the MVD-affected dog is well into heart failure (Stage C) or even in Stage D before starting S/V, and for up-titrating the dosages.
 The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
Reported side effects of Entresto include:
• Diarrhea
• Vomiting
• Drop in blood pressure
• Weakness
• Difficulty standing
There is a Facebook group, Entresto for dogs with CHF/MVD - sharing stories and what we have learned, which consists of hundreds of members who discuss their dogs' experiences with Entresto.
RETURN TO TOP
--- amlodipine in Stage C
Amlodipine besylate (Amodip, Norvasc) is a long acting vasodilator, meaning that it can decrease blood prssure by relaxing smooth muscles and widening blood vessels, allowing more blood to flow through them. Unlike pimobendan, it does not also have inotropic effects but as a vasodilator, it is much more potent than pimobendan and also considerably less expensive. Amlodipine acts by calcium channel blockage. It decreases resistance to the forward flow of blood, which results in a decrease in the back flow of blood from the left ventricle through the mitral valve into the left atrium (mitral regurgitation), and thereby decreases pressure in the left atrium. High doses of amlodipine are necessary for it to be effective, up to 1 mg/kg/day. However, initial dosages normally start at around 9.1 mg/kg/orally per day. Blood pressure checks before starting this drug, and over the course of administrating it (as doses may need to be increased), are necessary.
Concurrent ACE-inhibitor medication is advised, as there is some evidence that at high dosages, amlodopine may activate the RAAS in healthy dogs.
In a June 2003 study of sixteen dogs in Stage C with moderate to severe mitral regurgitation (MR), treatment with amlodipine reduced systolic blood pressure (9.6% decrease) and severity of MR (15.1% decrease in regurgitant fraction) and the dimension of the left ventricle (3.5% decrease in LV end-diastolic dimension). However, no significant change of the echographic LA/Ao-ratio was detectable in that study. The investigators concluded that the drug can be used simultaneously with standard cardiac therapy, which would include ACE-inhibitor, Lasix, spironolactone, and digoxin in Stage C. Amlodipine reportedly may cause gingival hyperplasia with chronic administration in dogs, with a reported incidence of 8.5%.
See, also, this 2012 comparative study of amlodipine versus benazepril. In a 2011 study, the Canadian researchers concluded that:
"Combining furosemide, ACEI, pimobendan, spironolactone, and amlodipine may result in long survival times in dogs with MR [mitral regurgitation] and CHF."
However, combined use of CCBs and ACE-inhibitors is controversial because of concerns for systemic hypotension and worsening renal function. Also, overdoses of CCBs can cause serious adverse effects on the cardiovascular system. See this September 2015 report specifically on amlodipine.
In a May 2017 report of a study involving 21 MVD-affected dogs in Stage C, a team of Brazilian researchers compared the treatment of CHF dogs with either pimobendan or amlodipine. They found no statistically significant differences in measurements of systolic blood pressure, or electrocardiographic parameters, or diastolic function using echocardiographic parameters. They concluded that neither pimobendan nor amlodipine appears to be superior to the other. Because of those results, the invesigators speculated that the clinical benefits of pimobendan in Stage C of MVD may be due to its vasodilating properties rather than its inotropic effects.
In a February 2022 article, South Korean researchers found in one Yorkshire terrier with multiple disorders (hypertension, pancreatitis, Cushing's disease, Stage B1 of MVD, chronic kidney disease, and a previous cerebellar infarction (stroke), that a high dose of amlodipine (0.4 mg/kg PO q12h) was the cause of pleural effusion, which is the build-up of excess fluid between the layers of the pleura membranes outside of the lungs. The pleura line the lungs and the inside of the chest cavity, and normally, there is only a small amount of fluid in the pleura. When the dosage of amlodipine was reduced to 0.3 mg/kg PO q12h, the symptoms of pleural effusion disappeared within 24 hours, and by the fifth day, no pleural effusion was identified on x-rays.
In an April 2022 article, Korean veterinary cardiology researchers added the calcium-channel blocker amlodipine to the current medications of 24 small breed dogs (none being cavalier King Charles spaniels) all diagnosed with Stage B2 or C of mitral valve disease (MVD), for a period of 7 days. They found no adverse effects, and that several measurements of MVD, including left ventricular end-diastolic internal diameter (LVIDD), left ventricular end-diastolic diameter normalized for bodyweight (LVIDdN), left atrial diameter (LAD), and E wave reduced statistically after the week of amlodipine treatment. The LVIDdN were decreased by 8% (2.2 mm); the LA/Ao ratio, was decreased by 6.4% (1.57 mm); the E wave was decreased by 14% (0.18 m/s). They concluded that: "These findings suggest that low-dose amlodipine should be considered as treatment for dogs with ACVIM stage B2-C MMVD."
See this September 2015 Plumb's overview for more details.
RETURN TO TOP
--- isosorbide dinitrate (ISDN) & arteriolardilators
Isosorbide dinitrate (ISDN) is a cavernous artery vasodilator which leads to a reduction in preload and cardiac oxygen consumption. ISDN causes a decrease in arterial pressure and reduction in afterload. In a September 2013 article involving experimentally induced MVD in six lab Beagles, the investigators report that ISDN significantly reduced left atrial pressure (LAP). The efficacy of ISDN currently is being studied by Dr. Dennis Trafny at Animal Medical Center in New York. See this article.
Sodium nitroprusside (Nitropress, Sodium Niroprusside) is an intravenous drug used to control high blood pressure in CHF dogs. It is a smooth muscle relaxant which causes vasodilation of the arteries and veins. It requires almost constant, close attention to the blood pressure levels.
Other arteriolardilators include milrinone (Primacor), hydrazaline (Apresoline), levosimendan (Simdax), and SCH00013.
In a November 2019 article, Michigan State University veterinarian reported the safe use of intravenous nitroglycerin (IV-NTG) in treating three MVD-affected chihuahuas in heart failure (CHF) -- Stage C -- to reduce preload pressure on the left ventricle and improve blood flow. They elected to use IV-NTG due to the severity of the dogs' CHF conditions and lower cost of IV-NTG compared to nitroprusside, an extremely expensive drug. They report that these are the first reported cases of using IV-NTC on veterinary patients with naturally occurring MVD, and that no significant adverse effects were observed. All three dogs' CHF were resolved following treatment, although the success cannot be attributed to the IV-NTG because of the small number of cases and the other medications used to treat the dogs' conditions.
ITI-214 is an inhibitor of phosphodiesterase type-1 (PDE-1), one of eleven main phosphodiesterases. (For example, PDE-3 is the phosphodiesterase inhibited by pimobendan, thereby increasing cardiac contractility.) In a July 2018 article, medical researchers report finding that, "The combination of effects [of ITI-214] in both dog and rabbit results in a rise in cardiac output with negligible changes in cardiac preload and arterial systolic pressure." ITI-214 is a product of Intra-Cellular Therapies, Inc.
Digoxin* (Lanoxin, Digitek, Digox, Lanoxicaps, Cardoxin), a cardiac glycoside extracted from the foxglove plant (digitalis), may be used to improve heart muscle strength to help the heart contract more strongly. Hydralazine (Apresoline, BiDil) is another arteriodilator which may be prescribed.
*WARNING: Digoxin toxicity (digoxinemia) may occur if the AF patient also has pre-existing ventricular tachyarrhythmias or renal dysfunction, or hpokalemia (low potassium level in blood serum), or in combination with certain other drugs, including diltiazem and spironolactone. See this January 2022 book.
RETURN TO TOP
--- angiotensin receptor blockers (ARBs) in Stage C
Angiotensin receptor blockers (ARBs) are drugs intended to block activation of the renin-angiotensis-aldosterone system (RAAS) due to RAAS' known contribution to the development of CHF in MVD-affected dogs. ARBs include Sacubitril/valsartan (Entresto, Sacufox V, Vymada, LCZ696) discussed separately above, and:
•
telmisartan (Micardis)
• valsartan (Diovan)
Studies currently are underway or planned to determine if some of these ARBs will significantly lower the incidence of ABT than will treatment with ACE-inhibitors.
Telmisartan (Micardis): A typical initial dosage is 1 mg/kg orally every day. Blood serum contents of blood urea nitrogen (BUN), creatinine, sodium, potassium, and chloride should be checked before beginning and periodically afterwards. Telmisartan and other ARBs may have these uncommon side effects: gastrointestinal upset, weakness, lethargy, azotemia, hyperkalemia (excessively high potassium level), and hypotension (low blood pressure).
In an October 2018 article, Japanese researchers reported that the ARB telmisartan did not suppress RAAS activation in five healthy laboratory beagles.
In a December 2020 article, University of Pennsylvania researchers compared the effectiveness of angiotensin converting enzyme inhibitors (ACE-I) -- enalapril and benazepril -- with the angiotensin receptor blocker (ARB) telmisartan in offsetting the activatation of the renin-angiotensin-aldosterone-system (RAAS) in the kidneys of dogs in Stage B2 of mitral valve disease. Eight MVD-affected dogs were included in the study, all diagnosed with Stage B2 of MVD, including two cavalier King Charles spaniels (25%). They report finding that telmisartan treatment resulted in higher activity of favorable angiotensin peptide (AP) concentrations, as compared to ACE-I treatment. They concluded that:
"Treatment with ARBs, such as telmisartan, or combined ARB and ACE2 [angiotensin converting enzyme 2] treatment represents a potential treatment strategy for dogs with DMVD and warrants additional preclinical study."
In a December 2024 study of 36 dogs divided into 3 groups (D1 group - enalapril was used, D2 - enalapril and spironolactone, D3 - telmisartan), the telmisartan group was foound "effective for treating dogs with MMVD because it lowers blood pressure, blocks angiotensin II receptors and improves hemodynamics, reducing the strain on the kidneys and myocardium."
RETURN TO TOP
--- alpha & beta blockers in Stage C
Alpha and beta blockers and alternatives include:
• Carvedilol (Coreg)
• Bisoprolol
• Nebivolol (Bystolic)
• Propranolol
• Phenoxybenzamine ( Dibenzyline)
• Atenolol
(Tenormin, Tenoretic)
• Nicorandil (Ikorel, Angedil, Dancor, Nikoran-PCA, Aprior,
Nitorubin, Sigmart)
Carvedilol (Coreg), bisoprolol, nebivolol (Bystolic), and propranolol also are being prescribed for Stage C MVD by some cardiologists. They are non-selective beta-and alpha-blockers with anti-oxidant effects -- also known as beta- (B-) adrenergic receptor antagonists (BARA) -- which reduce the heart's rate and the force of its contraction, thereby reducing the work of the heart. Also, in a 2009 study, it has been suggested that BARA have the potential to slow the progression of the mitral valve's degeneration by interfering with the serotonin signaling pathway -- a possible major factor in MVD progression -- and by reducing the "wear and tear" of the valve by reducing the pressure differences between the left ventricle and atrium. See also this January 2015 report regarding Carvedilol's effect upon hypertension.
Phenoxybenzamine ( Dibenzyline) is another alpha-blocker, with vasodilator properties.
A less expensive alternative beta-blocker is atenolol (Tenormin, Tenoretic). However, atenolol lacks the vasodilatory and antioxidant properties of carvedilol. Other beta blockers include metoprolol (Lopressor, Toprol). In a June 2021 article, Brazilian researchers reported that 30 days of metoprolol associated with at least 2 months of classic treatment for MVD-affected dogs in Stace C improved their quality of life and cardiac autonomic function.
Nicorandil (Ikorel, Angedil, Dancor, Nikoran-PCA, Aprior, Nitorubin, Sigmart), is an alternative to beta-blockers and/or calcium antagonists. It is a potassium channel activator with arterial vasodilator and venodilating properties. It increases blood flow through the heart, improves the heart's blood and oxygen supplies, and reduces its workload. Nicorandil is not available in the United States. See this January 2011 article on nicorandil.
RETURN TO TOP
--- omecamtiv mecarbil
Omecamtiv mecarbil (OM) directly targets the pumping function of the heart, by stimulating the protein cardiac myosin, which converts chemical energy into the force that makes the heart contract. OM is a therapeutic for the treatment of heart failure in humans and potentially in dogs. OM increases contractile strength and cardiac output without causing an increase in Ca2+ levels or myocardial oxygen consumption in humans and may have the potential to treat HF in veterinary medicine. However, OM has been shown to increase the oxygen demand of the heart.
In a June 2025 abstract, researchers reported on the COMPOSE Study, in which 6 healthy laboratory dogs were treated intravenously with a combination of pimobendan and omecamtiv mecarbil (OM) tp determine their safety and effectiveness in increasing canine myocardial systolic function. They report finding that the dogs' left verticular ejection fraction significantly increased with dual therapy compared to pimobendan or OM alone. They recommend future studies in dogs with mitral valve disease.
RETURN TO TOP
--- sildenafil and other hypertension medications
• Sildenafil (Viagra, Revatio) (a/k/a sildenifil)
•
Diltiazem (Cardizem, Diltstar, Tiazac, Tildiem)
• Bosentan (Tracleer)
• Hydralazine (Apresoline, BiDil)
• Phenoxybenzamine (
Dibenzyline)
• Ambrisentan (Letairis)
Primary pulmonary hypertension (pulmonary arterial hypertension -- PAH or PH) is not a common condition in dogs. However, secondary PH, as a complication of mitral valve disease, is more commonly diagnosed in dogs. PH is higher diastolic or systolic pulmonary arterial pressure than normal pressure. This high pressure may lead to increased right ventricular pressure and right atrial chamber enlargement, leading to possible right side heart failure.
This high pressure may lead to increased right ventricular pressure and right atrial chamber enlargement, leading to possible right side heart failure. In a March 2015 study of 212 dogs, including 30 cavaliers, diagnosed with either Stage B2 or Stage C MVD, the researchers found that 39% of the dogs had PH, and that PH was more commonly found in Stage C. They concluded that the presence of moderate to severe PH predicted a shorter survival time in dogs with MVD.
In an April 2018 abstract, UK cardiologists studied 94 MVD-affected cavaliers and 93 MVD-affected non-CKCSs, all in either Stage B2 or C, and found that cavaliers were more likely than non-CKCS to develop PH due to MVD, and that PH was associated with a greater likelihood of CHF and death (cardiac-related and all-cause mortality).
Sildenafil (Viagra, Revatio) (a/k/a sildenifil) a phosphodiesterase (PDI) 5 inhibitor, is being prescribed to lower pulmonary hypertension by some cardiologists for dogs with congestive heart failure, often in combination with pimobendan. In an October 2020 article, Thai researchers found that sildenafil, when added to conventional medical therapy for treating Stage C MVD-affected dogs with pulmonary hypertension aided in reducing systolic pulmonary artery pressure (sPAP).
The 2020 ACVIM Consensus Statement of guidelines for the diagnosis, classification, treatment, and monitoring of PH in dogs cautions that sildenafil or any other PDE5i pulmonary hypertension drug should not be prescribed as a first line treatment of PH secondary to MVD. Their reasoning is that, dogs with PH secondary to MVD have postcapillary PH. Postcapillary PH is a term given to left atrial (LA) or left ventricular filling pressure, which occurs most often in dogs with MVD that have increased LA pressure. Pimobendan has been found to lower LA pressure and thereby treat postcapillary PH. If, however, preliminary treatment with pimobendan and other MVD drugs does not relieve the PH, sildenafil may be added, and especially if the PH increases beyond the mild or moderate stages.
In an April 2006 French study report, tadalafil (Cialis), a long-acting PDI-5 inhibitor, belonging to the same family as sildenafil, has been shown to have decreased systolic pulmonary arterial pressure significantly. Another such pde-5 inhibitor is varenafil (Levitra). However, in a March 2012 article, researchers opined that "further studies are required to delineate the clinical effects and potential clinical value of these medications."
To the contrary, research by Dr. Rosemary A Henik, of the University of Wisconsin-Madison, has indicated that pulmonary venous hypertension due to left heart disease, is managed best with "afterload" reduction, and not sildenafil. More recently, a 2007 study by Drs. Joao S. Orvalho, William P. Thomas, and P. H. Kass found that "these data suggest that oral tadalafil, when added to conventional heart failure therapy, decreases the pulmonary artery pressure in this group of dogs."
Diltiazem hydrochloride (Cardizem, Diltstar, Tiazac, Tildiem) is a calcium channel blocker (CCB) medication used to treat hypertension in dogs. Calcium channel blockers, also called calcium antagonists, decrease the flow of calcium into muscle cells. It is similar to amlodipine. Another calcium channel blocker, clevidipine, which also lowers blood pressure is being studied at the Univeristy of California - Davis veterinary school, for its usefulness in treating MVD-affected dogs.
Bosentan (Tracleer) is a non-selective endothelin antagonist acted by blocking at the endothelin (ET)-1 receptor. In a 2000 Korean study, bosentan was found to have significantly lowered pulmonary arterial pressure in the treated dogs. In a 2000 US study, bosentan was given to dogs with moderate heart failure. The researchers found that long-term therapy with bosentan prevented progression of left ventrical (LV) dysfunction and reduced LV chamber remodeling.
Hydralazine (Apresoline, BiDil) is another arteriodilator which may be prescribed for high blood pressure. Phenoxybenzamine ( Dibenzyline) is an alpha-blocker with vasodilator properties, which also may be prescribed for high blood pressure.
Ambrisentan (Letairis) is an endothelin receptor antagonist (ERA) which has been approved by the FDA for treating human adults diagnosed with pulmonary arterial hypertension (PAH). In a May 2022 article, ambrisentan has been reportedly found to improve appetite, activity, and respiratory functions, and also improve partial pressure of arterial oxygen and the alveolar-arterial oxygen gradient, with no apparent side effects, in 5 dogs with severe pulmonary hypertension despite ongoing treatment with sildenafil.
Beraprost sodium is a chemically stable prostaglandin I2 analog, reportedly effective against PH because of its vasodilating effects on pulmonary and systemic arterial smoothmuscle cells, protective effects on vascular endothelial cells, inhibition of inflammatorycytokine production, and antiplatelet effects. In this August 2022 article, dogs' PH condition improved both clinically and echocardiographically. In dogs with post-capillary PH, LV function and systemic circulation significantly improved without significant increases in left heart size or left atrial pressure indicators. The researchers found that beraprost may improve both pulmonicand systemic circulation without left heart loading in dogs with post-capillary PH.
RETURN TO TOP
--- reducing and increasing plasma NT-proBNP
• Aldactazide
• Nesiritide (Natrecor)
B-type natriuretic peptide (BNP) is a natriuretic, diuretic, vasodilatory, and anti-remodeling hormone produced by myocardial cells in response to heart failure due to MVD (and other causes of heart failure). ("Natriuretic" means: causing the excretion of an excessively large amount of sodium in the urine.) BNP is activated during heart failure to counteract the effects of activation of the renin angiotensin aldosterone system (RAAS) in the heart, kidneys, and blood vessels. However, as the MVD progresses, the dog's ability to produce active BNP declines, lessening its ability to counter the effects of RAAS activation.
In a June 2015 abstract, PennVet researchers found that by tweaking medications (furosemide, enalapril, pimobendan) and adding Aldactazide, they were able to reduce the concentration of N-terminal pro-B-type natriuretic peptide (NT-proBNP), the peptide hormone discussed above, in dogs in CHF. They noted evidence in human patients with CHF that therapies reducing NT-proBNP improved their outcomes. Depending upon the results of periodic blood tesing over 21 days, if NT-proBNP was above 1500pmol/L in the test group, the researchers would increase the dosages of the medications. They reported :
"In conclusion, a pre-specified treatment escalation algorithm in dogs with stable CHF due to MMVD and with NT-proBNP >1500pmol/L resulted in significant decreases in plasma NTproBNP concentration over time. Serum BUN and creatinine measurements significantly increased over baseline in these dogs, suggesting that careful monitoring of renal function is necessary during therapeutic escalation. Further study is warranted to determine whether targeted reductions in NT-proBNP result in improved outcomes in dogs with CHF secondary to MMVD."
Oddly enough, on the other hand, in a May 2017 article, a team of US researchers which included two of the same PennVet investigators, discuss the feasibility, safety, and tolerance of injecting synthetic canine B-type natriuretic peptide (syncBNP) in four healthy dogs and two dogs with stage B1 mitral valve disease, including a cavalier. They also gathered data regarding the effect of syncBNP on plasma cGMP, kidney function, and RAAS. The found that syncBNP was feasible, well tolerated at all doses, and safe, and that it significantly increased median plasma cGMP. They recommended further studies using syncBNP for treatment of MVD.
In a January 2019 article, in which seven MVD-affected dogs (2 CKCSs) in CHF were tested, the researchers found that synthetic canine BNP1-32 produced no measurable beneficial cardio-renal effects, leading them to suggest that dogs with chronic CHF may have a reduction in natriuretic peptide responsiveness.
Nesiritide (Natrecor), the recombinant form of B-type natriuretic peptide (BNP1-32), the peptide hormone discussed above, has been shown in studies of induced severe CHF in dogs, when combined with the diuretic furosemide, to inhibit activation of aldosterone while maximizing natriuresis and diuresis and preserving renal function. See, for example, this 2004 report.
RETURN TO TOP
--- other drugs for treating Stage C
• Capadenoson
• Elamipretide (Bendavia, SS-31)
• Ivabradine
•
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors
Capadenoson is a partial adenosine A1 receptor agonist which has been shown in an April 2013 study to improve left ventricular function and prevent progressive heart enlargement in dogs in heart failure.
Elamipretide (Bendavia, SS-31) is a mitochondrially-targeted tetrapeptide that reportedly restores protein and mRNA levels in the left ventricle (LV) of dogs in heart failure (HF). In this May 2018 abstract, researchers at the Henry Ford Hospital report that mRNA and protein levels are decreased in LV of HF dogs and are normalized after chronic therapy with Elamipretide, resulting in observations of improved LV function and rate of ATP synthesis.
Ivabradine is a selective sinus node If Channel (funny channel) inhibitor which may be used to lower and maintain heart rate without affecting cardiac function. In this January 2017 article, ivabradine reportedly was found to be safely administered to dogs with congestive heart failure. See also this November 2017 abstract which reports finding that ivabradine lowers the heart rate in dogs, with no clinical discernable side effect, and therefore is safe to use in dogs with mitral regurgitation. In a May 2018 study of seven laboratory Beagles with naturally-occuring asymptomatic MVD (ACVIM Stage B), The researchers reported that a single oral dose of ivabradine for dogs with asymptomatic MVD reduced both the dogs' heart rate (HR) and the hearts' consumption of oxygen (MVO2) without significantly affecting blood pressure. They concluded that ivabradine may be promising for management of an elevated HR in asymptomatic MVD-affected dogs. In a September 2018 article, the same team of Thai veterinary researchers conducted a long term (3 months) study of oral doses of ivabradine in four MVD-affected Beagles with heart enlargement (Stage B2). They evaluated the dogs'heart rate (HR), blood pressure (BP), myocardial oxygen consumption (MVO2), and heart rate variability (HRV). They report finding that "the results revealed that chronic administration of ivabradine significantly decreased HR, BP, and RPP without adverse effects. All indices of time- and frequency- domain of HRV at M3 were significantly increased when compared with baseline values)." They conclude that "The findings of this study imply that long-term treatment with ivabradine at a dose of 1.0 mg/kg twice daily in dogs with asymptomatic DMVD stage B2 decreased the HR, BP, MVO2 and improves HRV. This makes ivabradine potentially promising for management of elevated HR and impaired HRV in asymptomatic dogs with DMVD stage B2."
In a January 2026 article, Thai researchers investigated the effect of long-term oral ivabradine on heart rate (HR), haemodynamics, cardiac function, and apoptosis (programmed cell death) in dogs with Stage B2 of MVD, meaning having a murmur and left-side heart enlargement but no fluid in the lungs (CHF). They treated 4 laboratory beagles in Stage B2 with oral ivabradine for 3 months. They found that the drug decreased the HR by 30.4% without significantly affecting systemic or pulmonary blood pressures, vascular resistance, or systolic/diastolic functions of the heart. They concluded that regular treatment with ivabradine lowered HR and reduced cardiac remodeling and apoptosis without impairing cardiac function in those dogs in Stage B2 of MVD.
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors (empagliflozin, dapagliflozin, velagliflozin, bexagliflozin) are human patient drugs used primarily for treating diabetes by lowering blood glucose and also cimproving kidney functioning and reducing inflammation and other aspects affecting MVD. In this July 2024 article, two cardiologists suggest that:
"Similarities between pathophysiological pathways leading to progression of cardiovascular and kidney diseases in human, canine and feline patients mean that SGLT2 inhibitors could provide benefits for companion animals beyond their use to control diabetes. Future clinical trials will determine whether this is the case and could well shed light on the relevance of different pathways for the beneficial effects of these drugs across species. Such trials would also need to assess the frequency of adverse events in veterinary patients with cardiovascular and kidney diseases to assess these against any benefits identified."
In this April 2025 article, the Japanese authors examined the pharmacology, safety, and pharmacokinetics of the SGLT2 inhibitors empagliflozin, canagliflozin, and dapagliflozin, and they concluded that dapagliflozin ithe the most favorable pharmaceutical in canines.
In a September 2025 article, the short-term effects of dapagliflozin, when added to conventional therapy (pimobendan, furosemide, and ramipril) for MVD-affected dogs in Stage C were studied in 5 dogs, none of which were cavaliers. Compared to a 7-dog control group, the dogs receiving dapagliflozin showed significant reductions in the percentage change from baseline for left atrial to aortic root ratio (LA/Ao), left ventricular internal diameter in diastole normalized to body weight (LVIDdN), end-diastolic volume, end-diastolic volume index, end-systolic volume, and end-systolic volume index. Additionally, ejection fraction significantly increased in the dapagliflozin group. Glucosuria was consistently present only in the dapagliflozin group. The investigators concluded that their findings suggested that dapagliflozin, when added to conventional therapy, may promote reverse remodeling and improve cardiac function in dogs with Stage C MVD.
RETURN TO TOP
--- bronchial dilators for coughing
Many veterinarians associate a dry, hacking cough with heart failure. They call it a "cardiac cough" and attribute it to fluid build-up in the lungs (pulmonary edema) due to CHF. In most all cases, however, the term cardiac cough is a misnoner because the true cause of the cough probably is respiratory related and completely independent of any heart disorder.
Some cardiologists may prescribe a bronchial dilator (bronchodilator), such as a methylxanthine, for example, aminophylline, millophyline, oxtriphylline, theophylline* (Corvental, Nuelin, Apo-Theo-LA, Theo-Dur), or terbutaline (Brethine, Bricanyl) which are human grade prescription medications which relax and open air passages in the lungs, making breathing easier.** A narcotic, hydrocodone bitartrate with homatropine MBr or guaifenesin (Hycodan, Hydromet, Tussigon, Mycodone), or butorphanol tartrate (Torbutrol, Stadol, Torbugesic) may be prescribed to suppress the coughing by affecting the brain's cough centers.
* Theophylline is a
PDE inhibitor which should not be given concurrently with pimobendan, which
is another PDE inhibitor, unless the combination of those two drugs is
carefully balanced.
** Fluoroquinolone antibiotics should not be given concurrently with any
methylxanthines.
The cough could be due to a combination of factors, which include the enlarged left atrium of the heart pressing against and compressing the left mainstem bronchus, but more likely to airway disorders independent of any relationship with the heart. It may even cause the trachea to collapse*.
* Trachea collapse also may be due to Brachycephalic airway obstruction syndrome (BAOS).
In a March 2012 article, USA researchers found no association between left atrial enlargement (LAE) and bronchial collapse in MVD-affected dogs with moderate-to-severe LAE and chronic coughing, but they did find that airway inflammation is common in dogs with airway collapse.
In a January 2019 article, a pair of experts concluded that a cough in the absence of rapid and labored breathing would indicate that it is due mainly to a respiratory disease rather than a cardiac disease. Coughing is a hallmark sign of bronchitis. Dogs with severe pulmonary edema can cough but coughing is much more common with primary lung disease. By severe pulmonary edema, they mean that the fluid has completely filled the lungs and also has started to fill the upper airway passages, as well. (The x-ray below shows the heart's enlarged left atrium impinging upon the left main bronchus.).
One of the most common -- and potentially dangerous -- misjudgements some veterinarians tend to make is to assume that such a cough indicates the onset of CHF and the need for immediate treatment with a diuretic. Cardiologist Dr. Michele Borgarelli has warned:
"It should be stressed that cough is a general clinical sign of respiratory disease and its presence in a dog with a murmur is not an indicator for starting treatment for CHF."
So if a dog has had a cough for months and unchanged and the dog is not being treated with a diuretic, the cough is very unlikely due to heart failure.
RETURN TO TOP
--- treating exercise intolerance and loss of muscle mass (cardiac cachexia)
General nutrition is very important. Cavaliers at this advanced stage may suffer severe weight loss, called progressive cardiac cachexia, and they should be fed any protein-rich palliative food to maintain muscle mass. Read about cardiac cachexia as a symptom of MVD here. See this January 2012 article about cardiac cachexia and the importance of maintaining body weight as a preventative measure.
In the ACVIM's 2019 Consensus Statement on diagnosing and treating MVD, the panel of cardiologists recommend maintaining an adequate calorie intake to minimize weight loss that often occurs in CHF. Specifically, they advise a maintenance calorie intake in Stage C of approximately 60 kcal/kg of body weight. They suggest warming food, mixing wet food with dry food, and offering a variety of foods, to encourage the affected dogs to eat.
Cardiologists may prescribe an appetite stimulant, such as mirtazapine (Remeron) or meclizine (Antivert, Bonine, Dramamine II, Driminate II), or capromorelin (Entyce).
In a November 2016 article
by a team of French cardiologists and specialists,
 they
reported on the use of a palatable high-energy complementary feed
composed of high-energy ingredients (glucose syrup, soybean oil and cod
liver oil), hydrolyzed animal proteins, vitamins and oligo-elements,
called
Nutri-Plus Gel, in five case studies.
they
reported on the use of a palatable high-energy complementary feed
composed of high-energy ingredients (glucose syrup, soybean oil and cod
liver oil), hydrolyzed animal proteins, vitamins and oligo-elements,
called
Nutri-Plus Gel, in five case studies.
Case Report 4 in that study was of a female 13-year-old cavalier (right) in Stage C2 (advanced heart failure) of mitral valve disease, which had lost 1.5 lbs. in the previous month and now weighed 11.5 lbs. She exhibited signs of fatigue, lack of appetite, and diarrhea. Her MVD treatment had been the standard four medicines for cavaliers in heart failure (diuretic, pimobendan, ACE-inhibitor, and spironolactone). They reported:
"Nutri-Plus Gel was also prescribed to stimulate appetite, limit the already considerable loss in body weight (10% of the initial weight in one month), and facilitate treatment compliance (six drugs in all, requiring eleven oral doses). During the follow-up visit one week later, the owner reported that the digestive and cardiac symptoms had disappeared (stage C1). One month later, a gain in weight was noted (200 g [0.44 lb.]). Two years after this episode, the dog was in good general condition and no change in the medical treatment was proposed, as the symptoms had not returned. As the animal's weight is now stable, Nutri-Plus Gel is recommended solely on demand, during phases of hyporexia (infrequent to date, according to the owner)."
In May 2016, the USA's Food & Drug Administration (FDA) approved the canine appetite stimulant Entyce (capromorelin). Capromorelin is a ghrelin receptor agonist which mimics the activity of ghrelin, a hormone which reportedly causes a sense of hunger.
In a May 2017 abstract, a team of researchers at Henry Ford Hospital in Detroit reported on the success of a new drug, Neladenoson bialanate hydrochloride (BAY1067197), a partial adenosine A1-receptor agonist, in improving the function of mitochondria in the skeletal muscles of dogs in heart failure. The drug company Bayer AG has been funding this project. In a November 2016 abstract, the researchers concluded that Neladenoson has beneficial effects on mitochondria function in dogs in heart failure. They report improvement in mitochondria function after treatment with Neladenoson within one hour after initiation of therapy.
Mitochondria are organelles in the muscle cells, which take in nutrients, break them down, and create energy rich molecules for the cell. As heart failure progresses in MVD-affected dogs, the mitochondria reduce in size, resulting in reduced capacity to create energy. See our discussion of exercise intolerance in our Symptoms section, for more information.
The researchers opine that treating dogs in heart failure with Neladenoson can potentially reduce or reverse exercise intolerance and skeletal muscle weakness.
Our Blog: "Your Stage C, MVD-affected cavalier is losing weight. What to do?"
RETURN TO TOP
--- natural alternatives
In addition to the natural alternatives to diuretics and ACE inhibitors described above, natural supplements which may help to strengthen and energize the heart of a dog with severe MVD include:
• Coenzyme Q10 (CoQ10) -- Read about CoQ10 here. Doses are 100 mg. twice daily for cavaliers and other small dogs.
• D-Ribose (Corvalen Ribose or Pure Encapsulations Ribose), also known as alpha-D-ribofuranoside, for MVD-affected dogs in Stage B2 or Stage C. Read about D-Ribose here as a supplement to strengthen the heart muscle and otherwise energize MVD-affected dogs with enlarged left atriums and/or left ventricles. Doses are of 1/4 to 1/2 teaspoon with meals, twice daily.
A good general health supplement for older dogs in congestive heart failure is N, N-Dimethylglycine (DMG). Vetri-DMG is a pure DMG product offered by Vetri-Science Laboratories of Vermont (www.vetriscience.com). DMG is said to support the immune system, promote oxygen utilization, improve cardiovascular function, support liver function, and support ocular health.
Holistic supplements should be taken only if prescribed by a licensed veterinarian who also is holistically trained in TCM. Holistic veterinarians are licensed veterinarians who, in addition to their conventional veterinary medical education, are further educated and trained and certified in holistic veterinary modalites. Search webpages for finding holistic veterinarians in the United States and Canada are located here, and in the United Kingdom, here.
RETURN TO TOP
--- avoid vaccines
 Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.)
Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.)
Some veterinarians recommend that dogs with advanced mitral valve disease not be vaccinated with the usual serums, including rabies, because of possible adverse reactions which might accelerate damage to the dogs' hearts. In such cases, the veterinarians will write letters to the county licensing authorities which require periodic vaccinations, and in many instances, the counties will accept the veterinarians' letters and excuse the dogs from having to be vaccinated.
A list of states in the United States which will accept letters from veterinarians, certifying that rabies vaccinations would interfere in the health of the dog is available on-line here: RabiesAware.org. Note that any dog not vaccinated for health reasons should be kept from coming into contact with potential sources of rabies, such as racoons and other wild animals. This would be true for other diseases for which the dog has not been vaccinated, as well, and in addition, the dog should avoid events where many other dogs would be present, such as dog shows and dog parks.
Dr. Larry Glickman, veterinary immunologist at Purdue University's veterinary school, wrote regarding cavaliers:
"Our ongoing studies of dogs show that following routine vaccination, there is a significant rise in the level of antibodies dogs produce against their own tissues. Some of these antibodies have been shown to target the thyroid gland, connective tissue such as that found in the valves of the heart, red blood cells, DNA, etc. I do believe that the heart conditions in Cavalier King Charles Spaniels could be the end result of repeated immunisations by vaccines containing tissue culture contaminants that cause a progressive immune response directed at connective tissue in the heart valves. The clinical manifestations would be more pronounced in dogs that have a genetic predisposition [although] the findings should be generally applicable to all dogs regardless of their breed."
RETURN TO TOP
---
hospitalization
 As
noted above, dogs with severe (acute) signs of HF -- Stage C1 -- require
hospitalization for stabilization. In cases of acute heart failure, the hospitalized dog
will be treated with sedation, oxygen, intravenous injections of diuretics and pimobendan.
Such hospitalization may last at least 24 hours and usually longer. The
length of time depends upon if and when is able to breathe normally in
room air without fluid in the lungs. Normal breathing is called eupnea.
To do so, the lungs must be relieved of the fluid from the left atrium,
whiich is the duty of the diuretics.
As
noted above, dogs with severe (acute) signs of HF -- Stage C1 -- require
hospitalization for stabilization. In cases of acute heart failure, the hospitalized dog
will be treated with sedation, oxygen, intravenous injections of diuretics and pimobendan.
Such hospitalization may last at least 24 hours and usually longer. The
length of time depends upon if and when is able to breathe normally in
room air without fluid in the lungs. Normal breathing is called eupnea.
To do so, the lungs must be relieved of the fluid from the left atrium,
whiich is the duty of the diuretics.
Fluid in the lungs reduces the dog's ability to transfer inhaled oxygen to the blood, resulting in hypoxemia (low level of oxygen in the blood). Thus, supplementary oxygen will be administered under high pressure (hyperbric) by either housing the dog in an oxygen cage (above, right) or a nasal tube, called a cannula. This is a version of "positive pressure ventilation" (PPV). Additional oxygen also will cause the pulmonary arteries to widen (vasodilate), thereby decreasing the afterload of blood in the left ventricle.
Such cages must be monitored to maintain adequate temperature (to prevent overheating) as well as humidity, in addition to controlling the fraction of "inspired" oxygen (FiO2) from a high of no more than 50 to 60% down to the normal level of 21% of the gases is air.
When supplementary hyperbric oxygen is administered at the hospital, care is taken to avoid oxygen toxicity, which may result if more than 50% supplemental oxygen is given for more than 12 to 24 hours. Oxygen toxicity may cause an undesirable release of inflammatory mediators, resulting in a sudden lung failure known as acute respiratory distress syndrome (ARDS).
The dog also will be lightly sedated, most likely with butorphanol, to counter any anxiety it may be experiencing from its condition.
Some drugs not discussed in any detail here -- such as nitroglycerin ointment (Nitro-Bid), sodium nitroprusside (NitroPress), dobutamine, and hydalazine (Apresoline) -- likely also will be administered during hospitalizations. Dobutamine is a synthetic balanced catecholamine which stimulates cardiac contractility and coronary blood flow without causing a significant change in vascular resistance.
RETURN TO TOP
-- Stage D -- end stage MVD
Follows the Pimobendan box just below.
RETURN TO TOP
A Few Words About Pimobendan (Vetmedin, Cardisure)
 Cavalier King Charles spaniels suffering from mitral
valve disease (MVD) received a dose of really good news in April 2007, when
the U.S. Food and Drug Administration approved the use of pimobendan to
treat dogs suffering from heart failure (HF), a particularly
common disorder in cavaliers. (See
FDA Approval Report.)
Cavalier King Charles spaniels suffering from mitral
valve disease (MVD) received a dose of really good news in April 2007, when
the U.S. Food and Drug Administration approved the use of pimobendan to
treat dogs suffering from heart failure (HF), a particularly
common disorder in cavaliers. (See
FDA Approval Report.)
Pimobendan (Vetmedin, Cardisure, Safeheart, Pimocard, Pimotab, Zelys, Fortekor Plus*) has been shown to improve the quality of life for dogs suffering from CHF due to MVD. Pimobendan technically is a benzimidazole pyridazinone derivative and is classified as an inodilator (a calcium sensitizer and phosphodiesterase-III inhibitor and a positive inotrope and arteriovenous dilator) which reportedly has a positive affect upon the force with which the heart muscle contracts, and also eases the resistance in the circulatory system by dilating blood vessels. Other inodilators are milrinone, levosimendan, and SCH00013.
* Fortekor Plus is a combination of benazepril and pimobendan.
 Often, the cavalier in the late stage of heart
failure suffers from a progressive deterioration of the quality of its life,
which is due to the combination of an inability to comfortably keep the dog
free from fluid congestion in its heart, lungs, and abdominal cavity,
together with enlarged heart chambers, lethargy, collapse, and deterioration
of its kidney and/or liver functions. Eventually diuretics, ACE inhibitors,
and other drugs no longer are able to remove enough of the fluids and
increase the supplies of blood and oxygen to the heart. At that point, often
the owner elects euthanasia, rather than to allow the dog to continue to
suffer.
Often, the cavalier in the late stage of heart
failure suffers from a progressive deterioration of the quality of its life,
which is due to the combination of an inability to comfortably keep the dog
free from fluid congestion in its heart, lungs, and abdominal cavity,
together with enlarged heart chambers, lethargy, collapse, and deterioration
of its kidney and/or liver functions. Eventually diuretics, ACE inhibitors,
and other drugs no longer are able to remove enough of the fluids and
increase the supplies of blood and oxygen to the heart. At that point, often
the owner elects euthanasia, rather than to allow the dog to continue to
suffer.
Pimobendan now may be called to the rescue. In addition to dilating the blood vessels like ACE inhibitors do, pimobendan increases the strength with which the heart muscle contracts, which improves the heart's efficiency to function as a pump, and increases the blood flow to major organs. It even has been shown, in some studies, to actually reduce the amount of backflow of blood through the mitral valve and reverse the enlargement of the heart chambers. It also reportedly makes the ill dog perk up as if from a caffeine-like rush, due to its purportedly neuro-stimulatory properties. And, it may be administered safely with diuretics, ACE inhibitors, and digoxin. The FDA report states that pimobendan "is indicated for use with concurrent therapy for heart failure (e.g., furosemide, etc.) as appropriate on a case-by-case basis." Furosemide is a diuretic.
Remarkably, pimobendan also has been shown to have fewer severe side effects than its main rival drugs, the ACE inhibitors benazepril (brand names Lotensin, Fortekor) and enalapril maleate (brand names Enacard, Vasotec). See ACE Inhibitors -- More Pluses Or Minuses? Many dogs with overt signs of advanced heart disease are reported by their owners to act as if they feel better and have improved activity tolerance within a few days of beginning pimobendan treatment. However, researchers report that the clinical improvement may not correlate with hemodynamic improvement. They opine that pimobendan may have a central nervous system effect that promotes a feeling of physical and mental well-being in dogs as demonstrated by other phosphodiesterase inhibitors, such as propentofylline. See this November 2007 article.

Before prescribing pimobendan, cardiologists may require an echocardiogram to measure the heart's contractility (the efficiency of the pump function of the heart). This precautionary test is recommended, because a negative effect reportedly has been instances of pimobendan improving the heart's pumping ability and contractility to the extent that the mitral valve's major chordae tendineae have been overworked and have actually ruptured, causing immediate death. Therefore, the drug should not be prescribed for dogs whose hearts have remained strong despite the MVD. Cavalier owners should never self-prescribe pimobendan for their dogs suffering from mitral valve disease.
Owners also should be wary of general practice veterinarians prescribing pimobendan without first consulting with a cardiologist about performing an echocardiogram to resolve the contractility issue. Also, pimobendan should not be prescribed by a non-cardiologist for CKCSs which are not in heart failure (HF). Researchers have reported severe adverse cardiac effects upon pimobendan-treated dogs not in HF, including increased blood backflow through the valve, heart enlargement, and deterioration of the chordae tendinae. We have found, now that pimobendan is available to the general practice veterinarians, that many of them are prescribing it without taking the precautions which would be instinctive to cardiologists and internal medicine specialists.

On the container of Vetmedin tablets, there is this warning:
"Contraindications: Vetmedin should not be given in cases of hypertrophic cardiomyopathy, aortic stenosis, or any other clinical condition where an augmentation of cardiac output is inappropriate for functional or anatomical reasons.
Warnings: Only for use in dogs with clinical evidence of heart failure."
Also, on Vetmedin's website, it has this warning:
"The safety of VETMEDIN has not been established in dogs with:
●Asymptomatic
heart disease
●Heart
failure caused by etiologies other than atrioventricular valvular
insufficiency or dilated cardiomyopathy
●Dogs
younger than 6 months of age
●Dogs
with congenital heart defects
●Dogs
with diabetes mellitus or other serious metabolic diseases
●Dogs
used for breeding or pregnant or lactating bitches."
And, on the vetmedin.com website, there are these "Adverse Reactions" warnings:

However, in a September 2016 article, an international team of veterinary cardiologists report that the administration of pimobendan to MVD-affected dogs with echocardiographic and radiographic evidence of heart enlargement results in prolonging the pre-heart failure period by approximately 15 months over non-treatment (a placebo), which represents a substantial clinical benefit. The is called the EPIC Study. (Note that this study was entirely funded by the manufacturer of pimobendan.)
The bottom line of the EPIC Study is:
(1) The dog must be in Stage B2 of mitral valve disease; AND
(2) Have a murmur of at least Grade 3; AND
(3) The dog must NOT
be on any other cardiac medication; AND
(4) An echocardogram must
be conducted and show valvular lesions of the mitral valve,
regurgitation through the mitral valve (MR) on the color Doppler
echocardiogram, and have echocardiographic evidence of left atrial
and left ventricular dilation; AND
(5) X-rays showing evidence
of enlargement with a vertebral heart size (VHS) greater than 10.5.
This essentially means that once the MVD-affected dog develops a Grade 3 murmur and an x-ray showing enlargement, the owner should have a board certified veterinary cardiologist perform the echo exam.
Pimobendan in solid formulation was developed by Boehringer Ingelheim GmbH, a German pharmaceutical company, and is marketed under the registered brand name "Vetmedin". In Europe and elsewhere apart from the United States, it has been studied since the late 1980s and prescribed by veterinarians since the 1990s. There, it is offered by EuroVet Animal Health BV, a Netherlands company, as "Cardisure". Pimobendan can be expensive. It has become a popular item on the underground drug market and on international Internet websites. As long as Boehringer Ingelheim holds the FDA's grant of marketing exclusivity in the U.S., it should not be expected to be sold as a reduced price generic drug. However, the Australian company, Luoda Pharma, has registered a liquid formulation of pimobendan.
Pimobendan is available through an accredited compounding pharmacy on the Internet, Premier Pharmacy Labs, Inc., in dosages different from the limited choices offered by Boehringer Ingelheim for Vetmedin, including flavored oral liquids and capsules sized to order. Veterinarians' prescriptions are required.
Several veterinary research studies of pimobendan have been published leading up to the FDA's report. In studies of dogs with mitral regurgitation, it has shown improved survival, heart and respiratory rate, and left atrial size, without evidence of arrhythmogenesis. It has been compared favorably with ramipril in a March 2005 study report. In a March/April 2006 study report, Texas A&M University Drs. Sonya G. Gordon, Matthew W. Miller, and Ashley B. Saunders find that "pimobendan is safe, well tolerated, and leads to enhanced quality of life in dogs with CHF secondary to...chronic valvular disease when used in combination with furosemide or other conventional therapies (e.g., angiotensin-converting enzyme inhibitors, digoxin)" and that "ongoing studies are evaluating its effects on mortality associated with chronic valvular disease." See Veterinary Resources for citations.
In a January 2007 article, UK cardiologists compared the affect of pimobendan versus ramipril on the vertebral heart score of 42 MVD-dogs in congestive heart failure, including 24 cavaliers. They found that for the first few months of the 21 dogs treated with pimobendan, their VHS heart size decreased, meaning a reduction in the heart enlargement, while during the same period, the 21 dogs treated with rampilril experienced a continued increase in their VHS heart size.
In July 2008, Drs. Haggstrom, Boswood, O'Grady and several others reported that in a comparison study of pimobendan and benazepril hydrochloride (the QUEST Study): "Pimobendan plus conventional therapy prolongs time to sudden death, euthanasia for cardiac reasons, or treatment failure in dogs with CHF caused by MMVD [myxomatous mitral valve disease] compared with benazepril plus conventional therapy." Of the 190 dogs, the median time to death (called the "endpoint") was 267 days for pimobendan and 140 days for benazepril. They concluded that "the benefit of pimobendan persisted after adjusting for all baseline variables. A longer time to reach the endpoint was also associated with being a cavalier King Charles Spaniel, requiring a lower furosemide dose, and having a higher creatinine concentration." In a September 2013 follow-up analysis of the same QUEST Study patients, the same researchers also found that the two medications resulted in similar quality of life during the study. However, they found that pimobendan conferred increased time before the progression of CHF and resulted in smaller heart size, higher body temperature, and less retention of water.
In an October 2013 report issued by Swedish cardiologists J. Haggstrom, P.F. Lord, K. Hoglund, I. Ljungvall, O. Jons, C. Kvart and K. Hansson, they studied 16 dogs in congestive heart failure (CHF) due to mitral valve disease, including eleven cavaliers, They compared pimobendan to benazepril and found that in dogs with CHF caused by MVD, pimobendan significantly reduces the heart rate (HR), left ventricle (LV) and atrium (LA) dimensions, heart rate-normalized pulmonary transit time (nPTT), and N-terminal proatrial natriuretic peptide (NT-proANP), and increases the ejection fraction, in comparison to benazepril. The reduction in heart size in response to pimobendan treatment in dogs with CHF is in agreement with previous studies, but reductions in HR, NT-proANP, and nPTT in response to pimobendan treatment have not previously been described in naturally occurring MVD.
The dangers of giving pimobendan to dogs not yet in heart failure
Pimobendan also is being considered for cavaliers just beginning to develop heart failure and even in earlier stages of MVD. In a September 2016 article, an international team of veterinary cardiologists report that the administration of pimobendan to MVD-affected dogs with echocardiographic and radiographic evidence of heart enlargement results in prolonging the pre-heart failure period by approximately 15 months over non-treatment (a placebo), which represents a substantial clinical benefit. (Note that this study was entirely funded by the manufacturer of pimobendan.)
To be included in the study, a dog had to be 6 years of age or older, have a mitral valve murmur of at least Grade 3 of 6, have echocardiographic evidence of advanced MVD consisting of characteristic valvular lesions of the mitral valve, regurgitation through the mitral valve (MR) on the color Doppler echocardiogram, and have echocardiographic evidence of left atrial and left ventricular dilation, plus radiographic evidence of enlargement with a vertebral heart size (VHS) greater than 10.5. The researchers warn that:
"... it should be borne in mind when interpreting these results that all dogs included in the analyses also met the echocardiographic inclusion criteria and these results therefore might not be generalizable to all dogs with a VHS > 10.5 in the absence of concurrent echocardiographic measurements and a confirmed diagnosis of MMVD."
Therefore, to apply the results of this study to future treatment of MVD-affected dogs, an x-ray of the heart, showing a VHS over 10.5, is not sufficient by itself, and that the two echo measurements must also be confirmed.
Of 354 dogs in the study, 161 (45.5%) were cavalier King Charles spaniels (CKCS). Of those 354 dogs, 178 of the dogs were treated with pimobendan, and 180 received the placebo. Of those 178 dogs treated with pimobendan, 59 (33.1%) reached congestive heart failure (CHF), 15 (8.4%) died of cardiac-related deaths during the treatment. The authors explained that:
"Although a greater number of dogs in the pimobendan group experienced spontaneous cardiac death (12 versus 5), the proportion of dogs in each group experiencing this event was not significantly different."
They concluded:
"Chronic oral administration of pimobendan to dogs with echocardiographic and radiographic evidence of cardiomegaly secondary to MMVD, in the absence of concurrent cardiovascular medication, results in the prolongation of the preclinical period, and is safe and well tolerated. The median time to the onset of CHF or cardiac-related death was prolonged by approximately 15 months, and the risk of a dog experiencing this event was reduced by approximately one-third; the majority of the benefit observed was attributable to delaying the onset of CHF. This substantial degree of prolongation of the preclinical period is f clinical relevance and is of importance to veterinarians and owners of dogs affected by this common disease."
So, the bottom line is:
(1) The dog must be in Stage B2 of mitral valve disease; AND
(2) Have
a murmur of at least Grade 3; AND
(3) The dog must NOT be on any other
cardiac medication; AND
(4) An echocardogram must be conducted and show
valvular lesions of the mitral valve, regurgitation through the mitral valve
(MR) on the color Doppler echocardiogram, and have echocardiographic
evidence of left atrial and left ventricular dilation; AND
(5) X-rays
showing evidence of enlargement with a vertebral heart size (VHS) greater
than 10.5.
This essentially means that once the MVD-affected dog develops a Grade 3 murmur and an x-ray showing enlargement, the owner should have a board certified veterinary cardiologist perform the echo exam.
It is troubling that the study report fails to note that
normal, heart-healthy cavaliers typically have VHS measurements as
high as 11.0, which is notably higher than the >10.5 criterion for
beginning pimobendan treatment. Considering that nearly half of the
dogs in the study were CKCSs, this oversight is rather inexplicable.
Also, the study's scientifically-baseless, species-wide definitions of left
atrial enlargement ((LA:Ao > 1.6) and left ventricle enlargement (LVIDDN
>1.7) necessarily include MVD-affected dogs with normal-sized hearts in
Stage B2 and other MVD-affected dogs with enlarged hearts in Stage B1.
It also is disappointing that the study does not go into any
details about those 8.4% of dogs being treated with pimobendan and
which died cardiac-related deaths while only in Stage B2. While the
authors do acknowledge that "concerns had previously been raised
about possible detrimental effects of the administration pimobendan
to dogs with preclinical MMVD", they provide nothing to explain
whether pimobendan played a role in those sudden and unexpected
Stage B2 deaths. To the contrary, they seem to be patting themselves
on the back for even including any data on these premature cardiac
deaths, stating:
"If our primary endpoint had focused exclusively on the onset of CHF, with dogs that died being censored, it might have appeared that we were choosing to ignore potentially detrimental effects of the treatment."Nevertheless, they minimize this 8.4% premature deaths of pimobendan-treated dogs by stating:
"We found, not unexpectedly, that only a small number of dogs met the primary endpoint in this way. Although a greater number of dogs in the pimobendan group experienced spontaneous cardiac death (12 versus 5), the proportion of dogs in each group experiencing this event was not significantly different."
Despite this "EPIC Study", great caution should be taken when considering treating any dog suffering only from mild, asymptomatic MVD. There is evidence from recent studies that treatment with pimobendan of dogs not in CHF may accelerate the symptoms of MVD, including increased regurgitation of blood through the mitral valve, deterioration of the chordae tendinea, and enlargement of the left side of the heart. Most recently, in the 2007 French study, "Comparative Adverse Cardiac Effects of Pimobendan and Benazepril Monotherapy in Dogs with Mild Degenerative Mitral Valve Disease: A Prospective, Controlled, Blinded, and Randomized Study", the researchers found (a) "increased systolic function in the PIMO group by comparison to baseline value as assessed by fractional shortening"; (b) "the maximum area and peak velocity of the regurgitant jet signal increased, whereas these variables remained stable in the BNZ group"; (c) "histologic grades of mitral valve lesions were more severe in the PIMO group than in the BNZ group"; and (d) "acute focal hemorrhages, endothelial papillary hyperplasia, and infiltration of chordae tendinae with glycosaminoglycans were observed in the mitral valves of dogs from the PIMO group but not in those of the BNZ group." They concluded:
"This study demonstrates that long-term administration of PIMO in dogs with asymptomatic MVD is associated with an increase in systolic function and, concomitantly, a progressive worsening of MVD with development of specific mitral valve lesions."
Bottom line: pimobendan can be hazardous to the health of cavaliers with MVD murmurs but no symptoms.
See also the 2005 study "Increased Mitral Valve Regurgitation and Myocardial Hypertrophy in Two Dogs With Long-Term Pimobendan Therapy", in which normal doses of pimobendan were determined to have increased mitral valve regurgitation and caused ventricular hypertrophy. Read also warning comments by Drs. Amara Estrada, Mark Rishniw, and George A. Kramer.
In a 2007 study, "Evaluation of Pimobendan in the Treatment of Early Mitral Valve Disease", the researchers concluded from their study of 26 dogs that their "data suggest a possible non-sustained positive inotropic effect and a reduction of the (mitral regurgitation fraction) at 90 days with the administration of pimobendan in early chronic MVD." They concluded, however, that more data are needed to further assess their findings. A positive inotropic effect means that the drug increases the strength with which the heart muscle contracts.
Dogs with CHF treated with pimobendan also have been found to live longer. In a July 2006 Swedish study of 76 dogs with acquired atrioventricular valvular disease, sponsored by Boehringer Ingelheim, the manufacturer of Vetmedin, which is pimobendan's brand name, researchers report that dogs treated with benazepril hydrochloride, an ACE inhibitor, lived an average of 128 days, while those treated with pimobendan lived an average of 415 days, a difference between four months and thirteen months. The study reportedly also found that within seven days of treatment with pimobendan, over half of the dogs were symptom free. Most of the dogs were treated concurrently with furosemide.
To the contrary, in an October 2006 report, University of Georgia internal medicine specialists Drs. Justin D. Thomason and Clay Calvert conclude that pimobendan may benefit dogs with congestive heart failure secondary to dilated cardiomyopathy or valvular insufficiency, only when used in conjunction with other cardiac drugs, such as ACE inhibitors.
Possible negative side effects of pimobendan include ventricular arrhythmias, particularly in dogs previously diagnosed with that disorder. In this 1989 article, researchers found "strong trends toward increasing incidences of sudden ischemic ventricular fibrillation in the presence of ... pimobendan. ... Hence, these findings suggest that with both positive inotropic agents ... sudden death may be increased via a reduction in ventricular refractoriness in the ischemically injured heart." However, in a 2007 study by Canadian Drs. M. Lynne O'Sullivan, Michael R. O'Grady, and C. Walker, which included eight cavaliers out of 23 dogs, they concluded that "Pimobendan did not result in an increase in frequency of ventricular arrhythmias in comparison to benazepril." A majorly significant side effect of pimobendan in human studies has been a high mortality rate. In the "Pimobendan in Congestive Heart Failure (PICO) trial", a 1996 study of 317 human patients, the researchers found that: "In both pimobendan groups combined the hazard of death was 1.8 times higher than in the placebo group."
In a study published in 2003 of the effect of pimobendan on rats, Spanish researchers found that "pimobedan ... administration [was] associated with an increased mortality from dysrhythmias." Similarly, in a June 2016 study, Thai researchers found that 10 of 19 dogs treated at least one month with pimobendan had developed cardiac arrhythmias, including atrial fibrillation, sinus tachycardia, second degree AV block, ventricular premature beat, and junctional premature beat. They warned that "cardiac arrhythmias may develop in some dogs treated chronically with pimobendan", and that "ECG monitoring should be performed in dogs chronically administered with pimobendan."
RETURN TO TOP
-- Stage D -- end stage of MVD
- Stronger diuretics
- Sacubitril/valsartan (Entresto)
- Appetite stimulants
- Bronchial dilators for coughing
- Oxygen
- Emergency room care
- Quality of life questionnaire
- Euthanasia
 Often, the cavalier in the late stage of congestive heart failure suffers
from a progressive deterioration of the quality of its life, which is due to
the combination of an inability to comfortably keep the dog free from fluid
congestion in its heart, lungs, and abdominal cavity, together with enlarged
heart chambers, lethargy, collapse, and deterioration of its kidney and/or
liver functions. Eventually diuretics, ACE inhibitors, pimobendan, and other
drugs, at standard dosages, no longer are able to remove enough of the fluids and increase
the supplies of blood and oxygen to the heart. This period of MVD is called
Stage D and also refractory CHF because
the standard dosages of medications prescribed for CHF no longer manage the MVD.
Often, the cavalier in the late stage of congestive heart failure suffers
from a progressive deterioration of the quality of its life, which is due to
the combination of an inability to comfortably keep the dog free from fluid
congestion in its heart, lungs, and abdominal cavity, together with enlarged
heart chambers, lethargy, collapse, and deterioration of its kidney and/or
liver functions. Eventually diuretics, ACE inhibitors, pimobendan, and other
drugs, at standard dosages, no longer are able to remove enough of the fluids and increase
the supplies of blood and oxygen to the heart. This period of MVD is called
Stage D and also refractory CHF because
the standard dosages of medications prescribed for CHF no longer manage the MVD.
At this stage, increased dosages or more powerful medications might be prescribed. For example, the diuretic furosemide will be increased (4 mg/kg q8h to q12h), and may be replaced by torasemide. Also, additional diuretics, such as hydrochlorothiazide (0.5 to 1 mg/kg q12h to q24h), may be added. Pimobendan dosages may be increased (0.9 to 1.2 mg/kg q24h). If sacubitril/valsartan (Entresto) has not been started in Stage C, it may be added in Stage D.
Additional vasodilators, such as amlodipine, may be prescribed. Digoxin or diltiazem may be added as anti-arrhythmic treatment. Sildenafil may be added to manage moderate or severe pulmonary hypertension. Periodic administration of oxygen may be necessary. (Photo is of is a Stage D cavalier receiving oxygen.)
In a June 2016 abstract, a team of Tufts University researchers reviewed 43 cases of dogs in Stage D of mitral valve disease (MVD). Nine of the dogs were cavalier King Charles spaniels. In summary:
• Median duration between diagnosis of congestive heart failure (CHF) and Stage D was 157 days.
• Median duration between diagnosis of Stage D and death was 311 days.
A good general health supplement for older dogs in congestive heart failure is N, N-Dimethylglycine (DMG). Vetri-DMG is a pure DMG product offered by Vetri-Science Laboratories of Vermont. DMG is said to support the immune system, promote oxygen utilization, improve cardiovascular function, support liver function, and support ocular health.
RETURN TO TOP
• stronger duretics
Torasemide (Isemid, Torsemide, Demadex, Tormis, UpCard), a loop diuretic, is more effective than furosemide due to its relatively consistent absorption and longer half-life. This typically results in torsemide having a longer duration and slower urinary excretion rate than does furosemide. Torasemide often is substituted for furosemide once furosemide seems to be losing its effectiveness (furosemide resistance) in Stage D. As an example, some cardiologists substitute torasemide once the patients need more than 8 mg/kg/day of furosemide. The initial dosage of torasemide typically is 0.15 to 0.2 mg/kg, by mouth every 12 to 24 hours.
* Azotemia is an abnormality consisting of abnormally high levels of urea, creatinine, and other nitrogen-rich compounds in the dog's blood.
Side effects due to torasemide include dehydration, lethargy, weakness, anorexia, azotemia*, electrolyte imbalances, and abnormal ranges for (blood urea nitrogen (BUN), creatinine, sodium, potassium, chloride, and blood pressure.
In a 2012 report, researchers compared doses of torasemide and furosemide in treating dogs with stable congestive heart failure (Stage C). They found that "torsemide is equivalent to furosemide at controlling clinical signs of CHF in dogs and is likely to achieve greater diuresis vs. furosemide." Torasemide is approximately 10- to 20-times as potent as furosemide in dogs, depending upon the frequency of the dosages -- 10-times stronger if given once daily and 20-times if given twice. (See this October 2015 article and this November 2019 article.) Therefore, the starting dosage is approximately 1/20 that of the furosemide dose. A possible side effect of torasemide is syncope. It also only requires a single dose per day for most dogs in Stage C.
In a November 2019 article, Colorado State Univ. researchers tested 6 healthy Beagles to compare the effects of the diuretics torsemide and furosemide upon activation of the kidney renin-angiotensin-aldosterone system (RAAS). Chronic RAAS activation is known to promote sodium and water retention, vasoconstriction, heart enlargement, and renal remodeling. The goal of the study was to compare the magnitude of RAAS activation between these two loop diuretics, administered at approximately equipotent dosages. Since torsemide has been found to be 20 times more potent than furosemide, the equipotent dosage of torsemide was 1/20th that of furosemide. The researchers report finding that, over a 10-day period, the diuretics behaved similarly, including the extent of increase in RAAS activity. There also was no significant difference in the extent of potassium excretion, although furosemide caused less than did torsemide. They also confirmed that torsemide given twice daily had a potency factor close to 20 times that of furosemide.
In an October 2017 article on the TEST Study, researchers sponsored by UpCard's manufacturer found that over a short-term of 84 to 91 days, torasemide resulted in a two-fold reduction over furosemide in the risk of reaching the cardiac endpoint (either a cardiac-associated death or worsening of the degree of CHF). They concluded by warning:
"However, given its potent diuretic effects, the lowest effective dosage should always be determined and, as recommended by the ACVIM consensus guidelines, dogs under such diuretic treatment should be closely monitored for renal and electrolyte abnormalities. Further studies are now required to explore the potential beneficial antifibrotic effect of torasemide on dogs with CHF, and its potential benefit over furosemide on long-term survival."
In an August 2020 article, a team of employees of Ceva Sante Animale, manufacturer of torasemide, along with PennVet cardiologist Mark A. Oyama, compared the diuretic to furosemide in a study of 319 MVD-affected dogs diagnosed with new onset congestive heart failure (CHF), including 52 (16.3%) cavalier King Charles spaniels. Their study, titled CARPODIEM, had the aim to evaluate the efficacy and safety of torasemide compared to furosemide in treating CHF. They report finding that torasemide was superior to furosemide in that it required only once-daily dosing, resulting in increased owner compliance, and also that torasemide had less than half the risk of cardiac death or euthanasia or worsening of CHF than did furosemide. They emphasized that monitoring of the dogs' reactions, hydration status, renal function, and serum electrolyte concentrations should be performed when using any diuretic.
In a May 2023 article, a team of Iranian researchers and one from Australia compared the effects of the loop diuretics furosemide and torsemide on certain echocardiographic measurements and blood pressure, in five healthy crossbreed dogs. They found that torsemide significantly reduced blood pressure an hour after administration, while furosemide did not, and also that torsemide increased heart rate above that of furosemide. They report finding no other significant differences between the treatment groups.
See also this November 2017 article on the efficacy of long-term use of torasemide.
Bumetanide, another loop diuretic, is 25 to 50 times more potent than furosemide. Otherwise, it performs in the same manner as furosemide. Proper dosages for treating pulmonary edema (fluid in the lungs) of dogs have not been published.
Acetazolamide is a carbonic anhydrase inhibitor, which can increase the excretion through the urine of sodium and bicarbonate, resulting in diuresis. It has been suggested for use as an additional diuretic for treating CHF in Stage D MVD-affected dogs, but no studies have been published.
Thiazide diuretics inlude:
• hydrochlorothiazide (Dyazide, Thiuretic, Esidrix, Hydrodiuril, Microzide)
• co-amilozide
or amiloride / hydrochlorothiazide (Moduretic, Moduret), a
combination of amiloride and hydrochlorothiazide.
A thiazide diuretic is typically administered in combination with a loop diuretic (e.g., furosemide) if the loop diuretic alone does not adequately control fluid retention. When added to the loop diuretic, dosages of hydrochlorothiazide range from an initial dose of 0.5 mg/kg orally every 24 hours to 2.0 mg/kg every 12 hours. Standard blood serum levels and blood pressure need to be checked periodically. Possible side effects include dehydration, azotemia, electrolyte imbalances, lethargy, weakness, and anorexia.
In a July 2021 article, a team of Japanese cardiologists studied the effects of adding hydrochlorothiazide (HTCZ) in treating 14 dogs in heart failure due to mitral valve disease (MVD) which no longer were responding to treatment with high doses of the loop diuretic torsemide. One of the 14 dogs was a cavalier. After dual treatment with the two diuretics, the echocardiographic data showed significant improvement. However, blood urea nitrogen and creatinine levels increased, and potassium levels decreased, indicating a decline in renal function following HTCZ administration. They concluded that their study suggested that the administration of HTCZ in combination with loop diuretics may improve cardiac function during advanced heart failure in MVD-affected dogs. However, as the combination of HTCZ and loop diuretics can deteriorate renal function, they advised that caution should be exercised prior to making recommendations regarding its use, and renal function should be monitored.
In a May 2025 article, Italian cardiology researchers examined the short-term and long-trm effects of hydrochlorothiazide (HCTZ) in 38 dogs, including 4 (10.5%) cavaliers suffereing from relapsing congestive heart failure (CHF) due to MVD. They report finding that, at a median of 7 days after adding HCTZ to the medication cocktail, creatinine, urea, and total calcium levels significantly increased, while sodium and potassium levels significantly decreased. While no dog developed severe electrolyte abnormalities, some dogs showed severe increases in creatinine and urea. After a median of 95 days, no significant echocardiographic changes developed. Episodes of CHF were more frequent before (median, one every 68 days) than after (median, one every 124 days) after startiing HCTZ.
In a September 2025 article, Italian researchers report results of a study of 25 MVD-affected dogs in Stage D (refractory congestive heart failure) treated with hydrochlorothiazide (HCTZ) as an additional diuretic. They started the HCTZ at 0.8 mg/kg every 48 hours and progressively increased the dosages to as high as 1.5 mg/kg per day. They observed azotemia (abnormally high levels of nitrogen-containing compounds in the blood) in 3 of the 25 dogs (12%). Azotemia can be due to insufficient or dysfunctional filtering of blood by the kidneys and can lead to uremia and acute kidney injury if not controlled. During the course of the study, 20 died, 18 due to MVD. The median survival time of the dogs was 268 days. Overall, they concluded that "HCTZ addition at the study dosages appeared clinically tolerated in most dogs with MMVD ACVIM stage D, with a prolonged median survival time for dogs in ACVIM Stage D."
RETURN TO TOP
• sacubitril/valsartan (Entresto)
 Sacubitril/valsartan
(Entresto, Sacufox V, Vymada, LCZ696) is a combination of valsartan, an
angiotensin
II receptor blocker
(ARB), which prevents vasoconstriction thereby lowering blood pressure
and improving blood flow. and sacubitril, a neutral endopeptidase (neprilysin -- NEP)
blocker, that prevents the breakdown of natriuretic peptides, which
helps lower blood pressure by reducing sodium levels. It is intended to promote diuresis and natriuresis and
counteract the effects of RAAS. It is being used as an alternative to
ACE-inhibitors in Stage C and D MVD-affected dogs. A dosage ranges from
5 to 10 mg/kg to 20 mg/kg orally every 12 hours.
Sacubitril/valsartan
(Entresto, Sacufox V, Vymada, LCZ696) is a combination of valsartan, an
angiotensin
II receptor blocker
(ARB), which prevents vasoconstriction thereby lowering blood pressure
and improving blood flow. and sacubitril, a neutral endopeptidase (neprilysin -- NEP)
blocker, that prevents the breakdown of natriuretic peptides, which
helps lower blood pressure by reducing sodium levels. It is intended to promote diuresis and natriuresis and
counteract the effects of RAAS. It is being used as an alternative to
ACE-inhibitors in Stage C and D MVD-affected dogs. A dosage ranges from
5 to 10 mg/kg to 20 mg/kg orally every 12 hours.
In an August 2018 article, a team of Auburn University researchers examined the effectiveness of sacubitril/valsartan (S/V) in 7 dogs diagnosed with mitral valve disease (MVD), compared to 6 MVD-affected dogs in the placebo group. They report finding that S/V is effective at lowering the urine concentration of aldosterone and that it was safe, with no deleterious effects on BUN, creatinine, and electrolyte concentrations, or systolic arterial pressure.
In a February 2019 article, the investigators compared the effects of sacubitril/valsartan versus just valsartan, and versus benazepril, on the dynamics of the renin-angiotensin-aldosterone system (RAAS) and the natriuretic peptides (NP) system in 18 laboratory beagle dogs after activating their RAAS with a low-salt diet over 15 days. They reported:
"In conclusion, the ARNI sacubitril/valsartan reduced ALD, a known risk factor of CV mortality, and enhanced the NP system via cGMPmediated pathways in a low-sodium diet model of RAAS activation. The results presented herein provide further evidence that the effects on the renin cascade extend to reduced ALD levels beyond that achieved with RAAS blockade alone. These positive findings in dogs also suggest that sacubitril/valsartan is a promising pharmacological candidate for increased survival in canine cardiovascular diseases."
In a July 2021 article, Thai investigators compared sacubitril/valsartan with the ACE-inhibitor ramipril in treating dogs diagnosed with Stage C (heart failure) due to MVD, along with both pimobendan and the diuretic furosemide. Twenty-one dogs -- none being cavaliers -- were divided into the S/V group (11 dogs) and the ramipril group (10 dogs) for four weeks. The investigators report that short-term administration of SV in dogs with MVD stage C resulted in a greater extent of reverse myocardial remodeling of both the left atrium and the left ventricle than in the ramipril group, as indicated by several echocardiographic parameters. They concluded:
"The current study suggested that the short-term effects of SV can reverse myocardial remodeling, as inferred from several echocardiographic indices (i.e., the reduction in LA/Ao, LVIDDN, EDVI and ESVI) in dogs with MMVD stage C. These findings would support the use of SV in clinically symptomatic heart failure in dogs."
However, they warn of a few limitations: (a) a very small sample size, with a much larger sample size needed to confirm the findings; (b) the study was for a very short term -- four weeks -- and a longer term study should be conducted; (c) the results thus far "do not suggest that ACEi should be replaced with S/V in dogs with MMVD stage C."
In a June 2024 article, Japanese resaerchers studied the effects of Entresto on the kidneys of 5 healthy laboratory dogs. They were given oral doses of 20 mg/kg. twice a day for 4 weeks. Kidney blood flow (renal hemocynamics) was assessed before the first dose and then on days 7 and 28. They report finding that Entresto "may enhance renal haemodynamics in healthy dogs."
In a June 2024 article, Japanese resaerchers studied the effects of Entresto on the kidneys of 5 healthy laboratory dogs. They were given oral doses of 20 mg/kg. twice a day for 4 weeks. Kidney blood flow (renal hemocynamics) was assessed before the first dose and then on days 7 and 28. They report finding that Entresto "may enhance renal haemodynamics in healthy dogs."
At the June 2025 ACVIM Forum, Dr. Justin Carlson presented a lecture, "Beyond the Guidelines: Sacubitril/Valsartan (ARNi) (Entresto)", in which he reported on 49 case studies of MVD-affected dogs in late Stage C or Stage D ("pretty bad cases that I thought were not going to do very well"), 7 of which were cavalier King Charles spaniels (and 7 Maltese and 6 Yorkies). Among those 49 patients in which he substituted Entresto (sacubitril/valsartan - S/V) for an ACE-inhibitor (such as benazepril or enalapril) and spironolactone, their mean survival time was 474 days, with a median of 381 days, and an overall survival range from 152 to 1,989 days. He compared those results to those of the VALVE Study trial and the QUEST Study. In the VALVE Study of Stage C dogs (in which the ACE-inhibitor ramipril was added to the combination of furosemide and pimobendan), the median survival time was only 214 days. In the QUEST Study (in which Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan alone. He emphasized that the patients in his group of 49 dogs being treated with Entresto were late Stage C or Stage D dogs, meaning that they were in far worse shape than the Stage C dogs just developing heart failure which were the subjects of the VALVE and QUEST studies.
Dr. Carlson said that the owners of 39 of his 49 patients (80%) reported their dogs had noticeably improved energy levels. The owners of 28 of the dogs (57%) reported that their dogs were coughing less. He found that "in a lot of these dogs", the S/V allowed for the safe reduction of diuretics, and in "a handful of these dogs", he has since stopped any diuretic medications, and "they are doing great." He reported that, over time, there was "a statistically significant difference" in the sizes of the left ventricle and left atrial ratio. He found in some dogs that over time their left atriums were back to normal in size.
As for adverse effects, he had only 2 of the 49 dogs with gastrointestinal distress. As for renal values, only one patient had significant worsening renal values, with 3 having increased azotemia in 4 months and 5 having significantly decreased renal values after 4 months. Regarding survival times, the mean survival time was 474 days, with a median of 381 days , and a range from 152 to 1,989 days. He pointed out that in the VALVE Study trial, in which an ACE-inhibitor (ramipril) was added to the combination of furosemide and pimobendan, the median survival time was only 214 days, and in the QUEST Study (in which the Stage C dogs received either pimobendan or benazepril), the median survival time was only 267 days for pimobendan. He emphasized that the patients in his group of dogs being treated with Entresto were Stage D dogs.
Dr. Carlson recommends "up-titration" of dosages of S/V, meaning starting at a low dosage and then increasing it, over 2 to 4 weeks, depending upon the dog's response and the blood test results. He stops both ACE-inhibitors and spironolactone when he starts the S/V, waiting 36 hours. His recommended dosage range in 15-30 mg/kg twice a day.
In a paper presented at the 2025 ACVIM Forum, cardiologists Ryan Fries, Elizabeth Malcolm, Sonya Gordon, and Justin Carlson wrote:
"When utilizing Entresto® in a group of canine patients (n=49) with congestive heart failure secondary to myxomatous mitral valvular disease or dilated cardiomyopathy, Entresto was well tolerated, with fewer than 5% of patients having undesirable side effects when titrated appropriately. While a FETCH questionnaire was not completed on patients, there was an overwhelming owner-noted improvement to the patients' quality of life when optimal dosing of Entresto was achieved based on owner report history during follow-up. Up-titration of the medication was done over the span of 1-6 weeks in most patients, with some patients requiring an extended up-titration over multiple months. Survival, echocardiographic, and dose-response data will be presented in a future manuscript. Based on the encouraging data from this cohort of patients, the optimal dosing, timing of up-titration, and ideal start time in patient disease stage warrant further study in a prospective manner."
 The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
The maker of Entresto warns humans that
ACE-inhibitors (e.g.,
enalapril, benazepril, etc.) should not be taken in
addition to Entresto. (See excerpt at right.) ACE-inhibitors
should be discontinued at least 36 hours before starting Entresto, and
some veterinary cardiologists recommend a "wash out period" of as long
as 72 hours before starting Entresto.
Reported side effects of Entresto include:
• Diarrhea
• Vomiting
• Drop in blood pressure
• Weakness
• Difficulty standing
• Kidney issues
There is a Facebook group, Entresto for dogs with CHF/MVD - sharing stories and what we have learned, which consists of hundreds of members who discuss their dogs' experiences with Entresto.
RETURN TO TOP
• appetite stimulants -- cardiac cachexia
General nutrition is very important. Cavaliers at this advanced stage may suffer severe weight loss, called progressive cardiac cachexia, and they should be fed any protein-rich palliative food to maintain muscle mass. See this January 2012 article about cardiac cachexia and the importance of maintaining body weight as a preventative measure.
In the ACVIM's 2019 Consensus Statement on diagnosing and treating MVD, the panel of cardiologists recommend maintaining an adequate calorie intake to minimize weight loss that often occurs in CHF. Specifically, they advise a maintenance calorie intake in Stage C of approximately 60 kcal/kg of body weight. They suggest warming food, mixing wet food with dry food, and offering a variety of foods, to encourage the affected dogs to eat.
Cardiologists may prescribe an appetite stimulant, such as mirtazapine (Remeron) or meclizine (Antivert, Bonine, Dramamine II, Driminate II), or capromorelin (Entyce).
In a November 2016 article
by a team of French cardiologists and specialists,
 they
reported on the use of a palatable high-energy complementary feed
composed of high-energy ingredients (glucose syrup, soybean oil and cod
liver oil), hydrolyzed animal proteins, vitamins and oligo-elements,
called
Nutri-Plus Gel, in five case studies.
they
reported on the use of a palatable high-energy complementary feed
composed of high-energy ingredients (glucose syrup, soybean oil and cod
liver oil), hydrolyzed animal proteins, vitamins and oligo-elements,
called
Nutri-Plus Gel, in five case studies.
Case Report 4 was of a female 13-year-old cavalier King Charles spaniel (right) in Stage C2 (advanced heart failure) of mitral valve disease, which had lost 1.5 lbs. in the previous month and now weighed 11.5 lbs. She exhibited signs of fatigue, lack of appetite, and diarrhea. Her MVD treatment had been the standard four medicines for cavaliers in heart failure (diuretic, pimobendan, ACE-inhibitor, and spironolactone). They reported:
"Nutri-Plus Gel was also prescribed to stimulate appetite, limit the already considerable loss in body weight (10% of the initial weight in one month), and facilitate treatment compliance (six drugs in all, requiring eleven oral doses). During the follow-up visit one week later, the owner reported that the digestive and cardiac symptoms had disappeared (stage C1). One month later, a gain in weight was noted (200 g [0.44 lb.]). Two years after this episode, the dog was in good general condition and no change in the medical treatment was proposed, as the symptoms had not returned. As the animal's weight is now stable, Nutri-Plus Gel is recommended solely on demand, during phases of hyporexia (infrequent to date, according to the owner)."
In May 2016, the USA's Food & Drug Administration (FDA) approved the canine appetite stimulant Entyce (capromorelin). Capromorelin is a ghrelin receptor agonist which mimics the activity of ghrelin, a hormone which reportedly causes a sense of hunger.
RETURN TO TOP
• bronchial dilators for coughing
Dogs with severe flooding of the lungs should not be exerted in any way. Some cardiologists may prescribe a bronchial dilator (bronchodilator), such as a methylxanthine, for example, aminophylline, millophyline, oxtriphylline, theophylline* (Corvental, Nuelin, Apo-Theo-LA, Theo-Dur), or terbutaline (Brethine, Bricanyl) which are human grade prescription medications which relax and open air passages in the lungs, making breathing easier.** A narcotic, hydrocodone bitartrate with homatropine MBr or guaifenesin (Hycodan, Hydromet, Tussigon, Mycodone), or butorphanol tartrate (Torbutrol, Stadol, Torbugesic) may be prescribed to suppress the coughing by affecting the brain's cough centers.
* Theophylline is a
PDE inhibitor which should not be given concurrently with pimobendan, which
is another PDE inhibitor, unless the combination of those two drugs is
carefully balanced.
** Fluoroquinolone antibiotics should not be given concurrently with any
methylxanthines.
RETURN TO TOP
• oxygen
 The onset of acute pulmonary edema requires
immediate recognition and therapy, including oxygen treatment, in order to
save the dog's life. (See the
Darcy's Daily Blog entry dated 8/25/06 for details of symptoms requiring
oxygen treatment.)
The onset of acute pulmonary edema requires
immediate recognition and therapy, including oxygen treatment, in order to
save the dog's life. (See the
Darcy's Daily Blog entry dated 8/25/06 for details of symptoms requiring
oxygen treatment.)
Fluid in the lungs reduces the dog's ability to transfer inhaled oxygen to the blood, resulting in hypoxemia (low level of oxygen in the blood). Thus, supplementary oxygen may be administered under high pressure (hyperbric) by either housing the dog in an oxygen cage (lower left) or a nasal tube, called a cannula (lower right), or a mask (above right). This is a version of "positive pressure ventilation" (PPV). Additional oxygen also will cause the pulmonary arteries to widen (vasodilate), thereby decreasing the afterload of blood in the left ventricle.
 Portable oxygen tanks, oxygen concentrators, and oxygen
tent/cages are available for use at the dog's
home and during transport to the
emergency room. Oxygen equipment may require a prescription from the
cardiologist.
Pawprint Oxygen
offers portable oxygen tanks and Kruuse Buster ICU oxygen cages.
Amazon.com offers
Buster ICU cages and
oxygen concentrators.
Such cages must be monitored to maintain adequate temperature (to
prevent overheating) as well as humidity, in addition to controlling the
fraction of "inspired" oxygen (FiO2) from a high of no more than 50 to
60% down to the normal level of 21% of the gases is air.
Portable oxygen tanks, oxygen concentrators, and oxygen
tent/cages are available for use at the dog's
home and during transport to the
emergency room. Oxygen equipment may require a prescription from the
cardiologist.
Pawprint Oxygen
offers portable oxygen tanks and Kruuse Buster ICU oxygen cages.
Amazon.com offers
Buster ICU cages and
oxygen concentrators.
Such cages must be monitored to maintain adequate temperature (to
prevent overheating) as well as humidity, in addition to controlling the
fraction of "inspired" oxygen (FiO2) from a high of no more than 50 to
60% down to the normal level of 21% of the gases is air.
In some cases, at-home oxygen therapy equipment can be used daily to
provide concentrated oxygen to dogs in
 Stage
D for which diuretics alone are not entirely effective in removing
fluid from the lungs. The equipment includes the air-tight crate, such
as the Kruuse Buster
ICU oxygen cage, and an
oxygen conentrator.
Treatments typically are for 30 minutes twice a day. Since the air-tight
crate can become overheated,
a cooling pad and
a digital thermometer
are needed to maintain a tolerable temperature in the crate during the
treatment.
Stage
D for which diuretics alone are not entirely effective in removing
fluid from the lungs. The equipment includes the air-tight crate, such
as the Kruuse Buster
ICU oxygen cage, and an
oxygen conentrator.
Treatments typically are for 30 minutes twice a day. Since the air-tight
crate can become overheated,
a cooling pad and
a digital thermometer
are needed to maintain a tolerable temperature in the crate during the
treatment.
When supplementary hyperbric oxygen is administered, care is taken to
avoid oxygen toxicity, which may
 result if more than
50% supplemental oxygen is given for more than 12 to 24 hours. Oxygen
toxicity may cause an undesirable release of inflammatory mediators,
resulting in a sudden lung failure known as acute respiratory distress
syndrome (ARDS).
result if more than
50% supplemental oxygen is given for more than 12 to 24 hours. Oxygen
toxicity may cause an undesirable release of inflammatory mediators,
resulting in a sudden lung failure known as acute respiratory distress
syndrome (ARDS).
In cases of emergency trips to the veterinarian when a Stage D dog experiences rapid respirations, a portable oxygen kit is available with an oxygen tank capable of lasting about 20 minutes. Paw Print Oxygen offers these kits without requiring a veterinarian's prescription.
RETURN TO TOP
• emergency room care
 If an MVD-affected dog incurs an instance of severe cardiac failure
(refractory heart failure)
and is taken to a veterinary emergency room, the likely care would
consist of:
If an MVD-affected dog incurs an instance of severe cardiac failure
(refractory heart failure)
and is taken to a veterinary emergency room, the likely care would
consist of:
• Oxygen therapy: either by placing the dog in an oxygen cage or attaching an oxygen mask to insure that the dog inhales oxygen.
• Diuretic therapy: initially inject a large dose (bolus) of a diuretic, such as furosemide, and establish a constant-rate flow of the diuretic intravenously.
• Pimobendan therapy: inject pimobendan intravenously.
• Rest: provide the dog with a cage in which to rest while being treated and monitored.
• Possibly mechanical ventilation.
• Try to keep the dog eating and drinking.
• Monitoring: Provide continuous heart monitoring -- pulse, blood pressure measurements, electrocardiogram, and blood tests.
Fluid in the lungs reduces the dog's ability to transfer inhaled oxygen to the blood, resulting in hypoxemia (low level of oxygen in the blood). Thus, supplementary oxygen may be administered under high pressure (hyperbric) by either housing the dog in an oxygen cage (right) or a nasal tube, called a cannula. This is a version of "positive pressure ventilation" (PPV). Additional oxygen also will cause the pulmonary arteries to widen (vasodilate), thereby decreasing the afterload of blood in the left ventricle.
Such cages must be monitored to maintain adequate temperature (to prevent overheating) as well as humidity, in addition to controlling the fraction of "inspired" oxygen (FiO2) from a high of no more than 50 to 60% down to the normal level of 21% of the gases is air.
When supplementary hyperbaric oxygen is administered at the hospital, care is taken to avoid oxygen toxicity, which may result if more than 50% supplemental oxygen is given for more than 12 to 24 hours. Oxygen toxicity may cause an undesirable release of inflammatory mediators, resulting in a sudden lung failure known as acute respiratory distress syndrome (ARDS).
The dog also may be lightly sedated, most likely with butorphanol, to counter any anxiety it may be experiencing from its condition.
A strong vasodilator, such as sodium nitroprusside, may be injected into a vein to restabilize a dog with acute pulmonary edema.
If the hypoxemia patient does not respond to the traditional oxygen administration described above, more aggressive oxygen therapies are available.
• Positive-pressure ventilation (PPV) , a form of mechanical ventilation (MV), is forcing oxygenated air into the lungs under pressure when inhaling, thereby inflating the lungs and either aiding or replacing normal respiration and to maintain blood oxygen tension. In a September 2014 study of 10 dogs treated with PPV, the investigators concluded that "there is a relatively good over all prognosis for discharge from the hospital in dogs with CHF that require mechanical ventilation." However, complications from PPV may include ventilator associated pneumonia, ventilator associated lung injury, and significant financial expense.
• High-velocity nasal insufflation (HVNI) involves providing oxygenated air to the nasal passages (nares) at a higher rate of pressure than standard oxygen therapy. In a May 2025 article, HVNI was utiliazed on 12 dogs diagnosed with congestive heart failure due to MVD or dilated cardiomyopathy after failing success with standard oxygen therapy. HVNI was successfully discontinued in 10 of the dogs (83%) after a median period of 14 hours (range 2 hours to 22 hours), all of which survived until they were discharged.
Some drugs not discussed in any detail here -- such as nitroglycerin ointment (Nitro-Bid), sodium nitroprusside (NitroPress), dobutamine, and hydalazine (Apresoline) -- may be injected into a vein to restabilize a dog with acute pulmonary edema. Dobutamine is a synthetic balanced catecholamine which stimulates cardiac contractility and coronary blood flow without causing a significant change in vascular resistance.
If the dog also has right-sided heart failure, in which fluid has caused congestion in the gastrointestinal tract, oral medications may be less effective because of a decrease in the dog's ability to absorb them. In such cases, injections of a diuretic -- furosemide usually -- may help restabilize the dog.
RETURN TO TOP
• quality of life questionnaire
The Functional Evaluation of Cardiac Health Questionnaire is a set of 18 questions for MVD-affected dog owners to answer. It has been developed based on widely accepted clinical signs of heart disease in dogs. (See box below.) The questionnaire consists of 18 questions to be answered by the dog's owner, who grades the severity of symptoms on a scale from 0 to 5, in which 0 = few symptoms and 5 = several symptoms, with higher scores indicating a poorer health-related quality of life. The questionnaire has been found to be valid as an independent measure of evaluating aproaching death due to MVD. See this March 2017 article.

RETURN TO TOP
• euthanasia
At this stage of deterioration, inability to breathe, and suffering, the owner may elect euthanasia, rather than to allow the dog to continue to suffer.
RETURN TO TOP
-- Experimental drugs
--- serotonin blockers
One study involves blocking the cavalier's excessive production of serotonin. Research thus far has suggested that: (a) serotonin (5HT) activates growth activity in canine mitral valves; (b) dogs with MVD have more serotonin receptors in their valve cells than other dogs; (c) mitral valve cells have the capacity to make their own serotonin; and (d) cavaliers also have a higher level of serotonin in their bloodstream. See, e.g., this June 2011 report.
Researchers are exploring the possibility that if serotonin levels can be reduced, by blocking the receptors in the mitral valves, then the progression of the deterioration of the valves and their leaflets can be slowed. One existing drug, approved for use by humans in Europe, is being tested on dogs with MVD to determine its effectiveness in reducing the level of serotonin and slowing the progression of MVD in cavaliers.
These drugs may include ketanserin (Ketensin, Vulketan, Sufrexal), a 5HT-R2A receptor blocker, or GR55562 dihydrochloride, a 5HT-R1B receptor blocker, based upon suggestions made by Dr. Mark Oyama in his September 2009 report and January 2010 report. See also this 1985 report about ketanserin.
In a December 2017 article, a team of human and veterinary researchers, including Dr. Oyama, studied the relationship between serotonin receptors and mitral valve interstitial cells (MVICs) and leaflet remodeling in humans and cavalier King Charles spaniels. Mitral valve specimens from four deceased cavaliers with severe mitral valve prolapse (MVP) and mitral regurgitation and four normal controls showed that serotonin 5HT receptors (5HTRs) signaling contributes to MVP deterioration, in particular, 5HTR2B upregulation. While this was primarily a study of human MVP, it indicates a possible relationship between the upregulation of 5HTR2B in cavaliers and the progression of mitral valve deterioration. It also shows that a 5HTR2B antagonist -- a drug called LY 272015 hydrochloride -- reduces MVICs activation in humans and mice. Since the cavaliers in this study all were deceased, the application of LY 272015 to dogs was not part of this study. LY 272015 is a beta-carboline derivative drug developed by the Eli Lilly company which is known to act as a potent and selective antagonist at the serotonin 5-HT receptor.
RETURN TO TOP
Surgery
- Cardio-pulmonary by-pass (CBP)
- Transcatheter edge-to-edge device (TEER) - V-clamp
- Transapical beating heart mitral valve replacement
- Left atrial decompression (LAD)
- Other minimally invasive surgeries
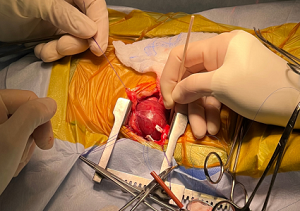 Replacement of the defective mitral valve is available in
veterinary medicine and has been for several years. However, surgical replacement usually
is cost-prohibitive (in a price range of $30,000 to $45,000) and would
require that the dog's renal system and other vital organs be in ideal
condition. Also, surgeries which include inserting mechanical devices into
the heart, must overcome innate reactions by the dog's systems,
including blood clotting (thrombosis) and the development of layers of
fibrovascular tissue or granulation tissue (pannus formation) around the
device.
Replacement of the defective mitral valve is available in
veterinary medicine and has been for several years. However, surgical replacement usually
is cost-prohibitive (in a price range of $30,000 to $45,000) and would
require that the dog's renal system and other vital organs be in ideal
condition. Also, surgeries which include inserting mechanical devices into
the heart, must overcome innate reactions by the dog's systems,
including blood clotting (thrombosis) and the development of layers of
fibrovascular tissue or granulation tissue (pannus formation) around the
device.
Therefore, MVD typically is treated by managing heart failure with medications. The goals of the veterinary cardiologist are to improve the dog's quality of life and to increase the length of its life.
• Cardio-pulmonary By-pass (CPB) Surgeries
- Dr. Masami Uechi -- Japan
- Dr. Katsuhiro Matsuura -- Japan
- Dr. Katsuhiro Matsuura -- USA
- Dr. Dan Brockman -- UK
- Dr. Peter Modler -- Austria
- Dr. Poppy Bristow -- UK
- Drs. Karina Graham and Laurencie Brunel -- Australia
- Dr. Sabine Bozon -- France
 Most all mitral valve surgeries involve stopping the heart and
by-passing it using an external circuit connected to the carotid artery
and the jugular vein. The procedures are called cardiopulmonary
bypass (CPB) surgeries These surgeries may involve replacing
the the mital valve tendons (chordae tendineae) and/or the valve with
either (a) implants, such as pig or cow heart valves or mechanical
devices, or (b) inserting a ring-like device to surround the valve,
called an annuloplasty device, which pulls the leaflets together to
improve the valve's function and reduce backflow of blood through the
valve. This procedure of implanting these rings is called mitral
annulus plasty. See a typical annuloplasty device below at
left.)
Most all mitral valve surgeries involve stopping the heart and
by-passing it using an external circuit connected to the carotid artery
and the jugular vein. The procedures are called cardiopulmonary
bypass (CPB) surgeries These surgeries may involve replacing
the the mital valve tendons (chordae tendineae) and/or the valve with
either (a) implants, such as pig or cow heart valves or mechanical
devices, or (b) inserting a ring-like device to surround the valve,
called an annuloplasty device, which pulls the leaflets together to
improve the valve's function and reduce backflow of blood through the
valve. This procedure of implanting these rings is called mitral
annulus plasty. See a typical annuloplasty device below at
left.)
Usually in all such surgeries, the chordae tendineae are
 replaced,
most commonly with
polytetra-fluoroethylene (PTFE) and expanded
polytetra-fluoroethylene (ePTFE) implants, which are made of a carbon and
fluorine based synthetic polymer (Gore-Tex and
SoftForm) that is biologically inert and non-biodegradable in the
body. (See a typical annuloplasty device at left.) In a 2012 article, Japanese
veterinary surgeons report that using ePTFE "has excellent tissue
compatibility and durability and can be effectively used for canine mitral
valve repair."
replaced,
most commonly with
polytetra-fluoroethylene (PTFE) and expanded
polytetra-fluoroethylene (ePTFE) implants, which are made of a carbon and
fluorine based synthetic polymer (Gore-Tex and
SoftForm) that is biologically inert and non-biodegradable in the
body. (See a typical annuloplasty device at left.) In a 2012 article, Japanese
veterinary surgeons report that using ePTFE "has excellent tissue
compatibility and durability and can be effectively used for canine mitral
valve repair."
In an
April 2017 article, Japanese heart surgeons report case studies of
two small dogs (a Maltese and a Shi
 Zhu) surviving 9 and 7 years
following mitral valve plasty, showing the durability of ePTFE sutures
as artificial chordae and long term success of semicircular suture
annuloplasty. In another
April 2017 article, Japanese researchers compare the suitability of
ePTFE and polypropylene as artificial suture materials for chordal
reconstruction in mitral valve plasty in the dog. They conclude that in
their surgeries, ePTFE was superior.
Zhu) surviving 9 and 7 years
following mitral valve plasty, showing the durability of ePTFE sutures
as artificial chordae and long term success of semicircular suture
annuloplasty. In another
April 2017 article, Japanese researchers compare the suitability of
ePTFE and polypropylene as artificial suture materials for chordal
reconstruction in mitral valve plasty in the dog. They conclude that in
their surgeries, ePTFE was superior.
In a
June 2022 article, Japanese veterinary cardiac surgeons report the
successful mitral valve repair surgeries on six small dogs diagnosed
with mitral valve disease (MVD) of various stages. The cavalier was a 7
year old male in Stage C (congestive heart failure). The surgical
technique they used is called a combination of the "Modified Loop
Technique" and "De Vega Annuloplasty" (MODEL). The modified loop
technique consists of several pre-tied loops of expanded
polytetrafluoroethylene (ePTFE) threads, of various sizes to match 80%
of the sizes of the
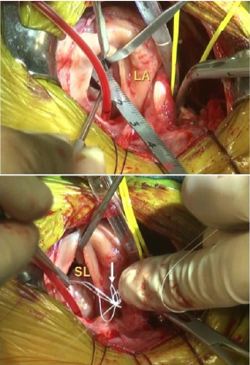 chordae
tendineae that would be encountered during the surgery. Three loops
serve as the replacement for each of the defective chords. (In the
photos at left, a ruler measures the length of the strut chordae. Then,
three loops [white arrow], each 80% of the length of the strut chordae,
were fixed to the papillary muscles and two of them are fixed to the
septal [front mitral] leaflet and one to the mural [rear mitral]
leaflet. Ao: Aorta; SL: Septal leaflet; ML: mural leaflet; LA: left
atrium.)
chordae
tendineae that would be encountered during the surgery. Three loops
serve as the replacement for each of the defective chords. (In the
photos at left, a ruler measures the length of the strut chordae. Then,
three loops [white arrow], each 80% of the length of the strut chordae,
were fixed to the papillary muscles and two of them are fixed to the
septal [front mitral] leaflet and one to the mural [rear mitral]
leaflet. Ao: Aorta; SL: Septal leaflet; ML: mural leaflet; LA: left
atrium.)
The De Vega annuloplasty technique involves sewing a continuous stitch of a single polypropylene thread suture in a purse-string fashion around half of the mitral valve so that when the thread is pulled at each end, the valve annulus can be tightened and reduced in size.The clinicians report that, after MODEL surgery, left atrial-to-aortic ratios (LA/Ao) significantly decreased from 2.20 ± 0.18 to 1.26 ± 0.22, and left ventricular end-diastolic internal diameter normalized to body weight (LVIDDN) significantly decreased from 2.03 ± 0.26 to 1.48 ± 0.20. In all cases, the clinical signs disappeared or improved significantly.
Atrial fibrillation is a possible complication following open heart surgeries. The term "post-operative new-onset atrial fibillation (POAF)" is applied to such cases.
Acute kidney injury may be a common side effect following cardiac-bypass surgeries in MVD-affected dogs. In a September 2022 article, a team of investigators at the Royal Veterinary College reported finding acute kidney injury (AKI) in 3 of 19 dogs (15.8%) which were in recovery following cardiac surgery under cardiopulmonary bypass (CPB). Of the 19 dogs, 7 were cavaliers (37%), although the report does not indicate which dogs developed AKI. Also, two of the dogs died during the post-operative period prior to discharge from the hospital. They report finding that specific kidney biomarkers -- inosine and urinary cystatin B (uCysB) -- changed significantly following the surgeries, potentially indicating tubular injury of the kidneys. They determined that MVD-affected dogs undergoing surgeries under CPB are "at increased risk of AKI", and that biomarkers may promptly identify AKI in order to implement preventative and theraputic treatments.
For a detailed description of the cardio-pulmonary bypass procedure, see the Mighty Hearts Project website.
RETURN TO TOP
•Dr. Masami Uechi -- Japan
 Japanese veterinary heart surgeon Dr. Masami Uechi (right)
and his team at JASMINE Veterinary Cardiovascular Medical Centre in
Yokohama, Japan, have been conducting open-heart mitral valve plasty
surgeries on small dogs since 2006. His clinic reports that through
2023, it has performed 447 surgeries with a 97% survival rate.
Japanese veterinary heart surgeon Dr. Masami Uechi (right)
and his team at JASMINE Veterinary Cardiovascular Medical Centre in
Yokohama, Japan, have been conducting open-heart mitral valve plasty
surgeries on small dogs since 2006. His clinic reports that through
2023, it has performed 447 surgeries with a 97% survival rate.
Mitral valve plasty involves suture repairs to the mitral valve leaflets and includes the insertion of artificial chords made of a polymer, expanded polytetrafluoroethylene (e-PTFE). They report in a 2009 journal article, "Mitral Valve Plasty in 11 cavalier King Charles Spaniels", that "these results suggest that mitral valve plasty is beneficial in CKCS with MVD."
While two of their CKCS patients died during the post-operative study due to complications, and three were diagnosed with syringomyelia, the researchers found that among the nine survivors, "at 1 and 3 months after surgery, the left atrial to aortic root diameter ratio ... and the plasma atrial natriuretic peptide level ... were lower than those before surgery ... There were also significant improvements in the number of prescribed cardiovascular drugs 1 month after surgery ... and in the cardiac murmur grade ... ."
Any cavalier owners interested in contacting JASMINE about having their dog surgically cared for should have their dog's cardiologist email Dr. Uechi at muechi@jasmine-vet.co.jp with the following information:
Dog's name:
Breed:
Body weight:Age:
F/M:
MVD Stage:
Current Medicines:
Quarantine end date:
Other underling medical conditions:
Owners email address:
Include these medical records: (1) Echocardiogram videos and still images in AVI or DICOM format (via dropbox or google drive would be fine; (2) Latest echo report with value ( LA/Ao, LVIDd…etc.; (3) Chest Xrays; (4) Abdominal ultrasound report (used to rule out potential surgical risks) report only and no image is needed.
If there are any other items theye need after they review those records, theye will ask the cardiologist what additional tests or information is needed by emailing him/her directly.
Also have your Veterinarian CC you on all communications so you are aware of the current progress.
Here is a chronological summary of Dr. Uechi's reports and veterinary journal articles on these surgeries:
Read a 2010 report and a 2012 report with updates of their surgeries.
In a 2011 article by Dr. Uechi, he reported that in mitral valve repair surgeries of 50 dogs with heart enlargement, overall the heart rate decreased and mitral regurgitation reduced, resulting in reduction of the enlargement of the hearts.
In a March 2012 report, Dr. Uechi stated that open-heart mitral valve repair consisting of installing a ring around the valve, a procedure called mitral annuloplasty, and/or replacing chordae tendineae with durable, artificial chordae have improved long-term clinical outcomes in small breed dogs, without the need for long-term anti-clotting therapies. He reported that post-operative improvements have included reduced regurgitation, decrease in heart size, reduction in murmur grade, improved appetite, elimination of cough, dyspnea, and anorexia. In a July 2015 article, Dr. Uechi reported open-heart surgeries on 370 dogs between 2006 and 2014. He wrote:
"After MVR [mitral valve repair], the heart rate significantly decreased from 118-164 bpm to 75-138 bpm. The grade of cardiac murmur was significantly reduced to 0/6-3/6, three months postoperatively, and the cardiac silhouette was reduced (VHS 9.8-11.5) in the chest X-rays. Echocardiography confirmed the marked reduction in both the mitral regurgitant ratio (62-87% to 4-64%, P<0.05) and the left atrial dimensions (LA/Ao 1.2-2.2). Mitral valve repair reduced cardiac size by reduction of the regurgitant rate. After surgery, clinical signs improved and patients were discharged within 12 days post operatively. Several dogs died within 10 days after surgery as a result of bleeding or pancreatitis. In the postoperative physical examination, cough was no longer present, and the animal's appetite had improved. In addition, with the improvement in general condition, body weight increased. The clinical signs had essentially disappeared by 1 month after surgery. In addition, cardiac reverse remodelling was also observed at 1 month post operatively. Based on the reduction in class of The International Small Animal Cardiac Health Council classification, the clinical signs had improved after MVR. In addition, the number of medications used decreased by 1 month post operatively. By 3 months after the surgery, many dogs did not require medication."
In a May 2012 report on 48 surgeries, Dr. Uechi and his team reported success in 45 cases of mitral annuloplasties and replacing the chordae tendineae with ePTFE.
In a June 2017 article, Dr. Uechi and his team reported that in a study of 65 dogs, including 12 cavaliers, severe mitral regurgitation (MR) was detected in 38 dogs before the surgeries, but in none following the surgeries. They concluded that mitral valve repair in dogs decreased MR volume, and that quantifying the MR volume is an effective method for evaluating the success of mitral valve repair.
In an August 2020 article, Dr. Uechi summarized his team's current techniques of surgical repairs to dogs' mitral valves. He stated that, "High survival rates can be expected in dogs if the appropriate surgery is performed." He has found that optimal timing of the surgery does not depend upon the stage of the MVD.
He described each part of the mitral valve and how it is repaired. These parts include the septal (anterior) leaflet, the mural (posterior) leaflet, the chordae tendineae (chords), and the papillary muscle. The septal leaf is repaired by inserting r4 to 8 pairs of artificial chords. If the septal leaf itself needs additional work, suture techniques are used to lengthen and straighten it. The mural leaf is repaired by adding 1 or 2 pairs of artificial chords and if necessary, sutures. Obtaining the normal shape of the mitral valve "is crucial", and that is done by mitral annuloplasty -- inserting a ring around the valve. Common complications include thrombus (blood clot) and hematoma (the collection of blood outside of a blood vessel). He suggested that dogs may be more susceptible to thrombus formation upon exposure to prosthetics than humans. Continuous suture method with ePTFE sutures is used, and limiting the exposure of blood to artifical surfaces. He stated that "Recently, however, the incidence of thrombosis in annuloplasty by mitral annuloplasty using continuous suture with ePTFE sutures has decreased to approximately 5%."
Hematoma of the left ventricular wall is a critical post-surgical complication. Hematoma may cause premature ventricular contraction, low blood pressure and, in some cases it can lead to death. Although the cause remains unknown, it is reasonable to suspect that ventricular extrasystoles and the persistence of hypotension after surgery are caused by left intraventricular hematoma. In left intraventricular hematoma with a significant decrease in blood pressure, left ventricular function can be recovered by reopening the heart and making an incision in the hematoma to reduce its size. Hematomas that do not affect blood pressure shrink in several days to several weeks. Other complications could include respiratory failure, anemia, and renal failure.
Dr. Uechi reported in January 2017 that he has performed MVD repair surgery on nearly 700 dogs over the previous 12 years, with an 85% survival rate. His contact information is: email: uechi.masami@cardiovets.jp; address: 2-7-3 Nakagawa, Tsuzuki, Yokohama, Kanagawa 224-0001 Japan; telephone: +81-45-532-8451; telecopier: +81-45-532-8456.
In a September 2014 paper before the WSAVA Congress, Dr. Isamu Kanemoto summarized the current methods of mitral valve surgeries and their pitfalls for small dogs. The primary difficulty in small dog surgeries is the need for miniaturization of devices and surgical tools. He wrote:
"There are two mitral valve operations for mitral regurgitation (MR); mitral valve plasty (MVP) and mitral valve replacement (MVR). In MVP, the self-valve is preserved and repaired. The advantage is no reaction of a foreign substance, though it requires the skilled techniques. On the other hand, the MVR technique is simple, but anticoagulant therapy is needed during life. As in humans, the first choice is MVP in dogs. ... On chordal reconstruction, shortening of prolonged chorda, and shifting of approximate chorda technique are very difficult in small dogs."
 In an
April 2017 article, Japanese veterinarians report on the success of
long term (90+ minutes) open heart surgeries of small dogs with severe
MVD.
In an
April 2017 article, Japanese veterinarians report on the success of
long term (90+ minutes) open heart surgeries of small dogs with severe
MVD.
A YouTube video of the actual open-heart mitral valve repair surgery is available here.
RETURN TO TOP
• Dr. Katsuhiro Matsuura -- Japan
 Since
2018, Dr. Katsuhiro Matsuura (right) and his team have been
performing mitral valve plasty on MVD-affected dogs, including
cavaliers, at the VCA Japan Shiraishi Animal Hospital in Saitama, Japan.
He uses a "loop-in-loop" technique to adjust the height of each mitral
leaflet. The procedure involves looping sutures through anchors attached
to the valve's papillary muscles, and passing additional sutures through
those loops, all at appropriate lengths for the valve's leaflets.
Since
2018, Dr. Katsuhiro Matsuura (right) and his team have been
performing mitral valve plasty on MVD-affected dogs, including
cavaliers, at the VCA Japan Shiraishi Animal Hospital in Saitama, Japan.
He uses a "loop-in-loop" technique to adjust the height of each mitral
leaflet. The procedure involves looping sutures through anchors attached
to the valve's papillary muscles, and passing additional sutures through
those loops, all at appropriate lengths for the valve's leaflets.
In a June 2022 article, Dr. Matsuura reported on the results of surgeries on 55 dogs, including 2 cavaliers, between July 2019 and November 2021. Fifty patients (90%) survived to discharge following surgery, with survival significantly decreased in Stage D dogs. Specifically, all 14 Stage B2 dogs (100%), 26 of 27 Stage C dogs (96.2%), and 10 of 14 Stage D dogs (71.4%) survived to discharge following their surgeries. A month following surgeries, there were significant reductions of overall heart size (vertebral heart score: preoperative 11.4 vs. post 1 month 10.2), left atrium (left atrium to aortic root ratio: preoperative 2.3 vs. post 1 month 1.5) and left ventricle (left ventricular end-diastolic diameter [normalized for bodyweight]: preoperative 2.2 vs. post 1 month 1.5), showing successful management of mitral regurgitation. All medications for mitral valve disease were discontinued 3 months after surgery. Recurrence of mitral regurgitation was not evident during the two-year follow-up period.
Dr. Matsuura's team in Japan may be contacted through this website.
RETURN TO TOP
• Dr. Katsuhiro Matsuura -- Gainesville, Florida USA
As of September 2023, Dr. Matsuura leads a team at the University of Florida's veterinary hospital in Gainesville, performing mitral valve plasty on MVD-affected dogs, including cavaliers. Details are available on the hospital's website at this link. As of February 2025, Dr. Darcy Adin reports that 52 dogs have received this surgery, with a 93% survival rate. The cost of this surgery as of that date is between $40,000.00 and $50,000.00 per patient.
RETURN TO TOP
• Dr. Dan Brockman -- United Kingdom
 Heart surgeon Dan Brockman (BVSc,
CVR, CSAO, MRCVS, Diplomate ACVS/ECVS) (right) at the Royal Veterinary College,
University of London, in England, has begun a animal cardiac surgery
program, which includes open-heart mitral valve surgeries on canines. He has
consulted with Dr. Orton at Colorado State University, and the two surgeons
have been working together to advance heart surgeries in the UK.
Heart surgeon Dan Brockman (BVSc,
CVR, CSAO, MRCVS, Diplomate ACVS/ECVS) (right) at the Royal Veterinary College,
University of London, in England, has begun a animal cardiac surgery
program, which includes open-heart mitral valve surgeries on canines. He has
consulted with Dr. Orton at Colorado State University, and the two surgeons
have been working together to advance heart surgeries in the UK.
Mr. Brockman may be reached at The Queen Mother Hospital for Animals, Hawkshead Campus, the Royal Veterinary College. For more information about RVC's open heart surgery program, email the team at qmhaheartsurgery@rvc.ac.uk The team's webpage is linked here.
In an April 2022 interview, Dr. Brockman answered questions about his team's mitral valve open heart surgery program. He said that RVC surgery center performs about two operations per month but is expected to increase that rate to one per week. The cost, which includes a pre-operative consultation, surgery, and one week of post-operative hospitalization is £12,000. His survival rate currently is 85% for dogs in Stage C and advanced Stage B2, and 90% since 2020, and 100% in 2022.
In an August 2024 article, Dr. Brockman reported that his team is dong two surgeries per week and plans to increase to three each week.
In a February 2025 article, Dr. Brockman (right) and his team of cardiologists and other specialists (in open heart surgeries of 132 canine mitral valve disease patients at the Royal Veterinary College in the UK between July 2015 and November 2022, reported on their procedures and success rates. Of the 132 dogs, 31 (23.5%) were cavalier King Charles spaniels. The short-term survival rate - meaning dogs discharged from the hospital following surgeries - was 81% (107/132), which included 26 of the 31 cavaliers (83.9%). They note that their short-term survival rate improved over time. Their article includes discussion of the strategies utilized in selecting candidates and their "learning curve" and organizational commitment to the program.
RETURN TO TOP
• Dr. Peter Modler -- Austria
 Dr. Peter Modler
(right) is performing surgical mitral valve repair on a routine
basis to dogs with advanced degenerative mitral valve disease. Thus far,
he has concentrated on treating cavalier King Charles spaniels in
congestive heart failure. His open-heart surgical procedure includes
installing a Gore-Tex annuloplasty ring around the valve, and also
replacing diseased valve chords with Gore-Tex, while the dog is on
cardiopulmonary bypass. Dr. Modler's clinic is located in Sattledt,
Austria.
Dr. Peter Modler
(right) is performing surgical mitral valve repair on a routine
basis to dogs with advanced degenerative mitral valve disease. Thus far,
he has concentrated on treating cavalier King Charles spaniels in
congestive heart failure. His open-heart surgical procedure includes
installing a Gore-Tex annuloplasty ring around the valve, and also
replacing diseased valve chords with Gore-Tex, while the dog is on
cardiopulmonary bypass. Dr. Modler's clinic is located in Sattledt,
Austria.
In a February 2015 report, Dr. Modler (right) reported on a case of mitral valve replacement surgery using Gore-Tex chordae and leaflets, which he performed on a 6 year old cavalier in congestive heart failure due to advanced MVD. In February 2017, Dr. Modler announced that he is performing surgical mitral valve repair on a routine basis to dogs with advanced degenerative mitral valve disease. Thus far, he has concentrated on treating cavalier King Charles spaniels in congestive heart failure. His open-heart surgical procedure includes installing a Gore-Tex annuloplasty ring around the valve, and also replacing diseased valve chords with Gore-Tex, while the dog is on cardiopulmonary bypass.
Dr. Modler's clinic is located in Sattledt, Austria. His contact information is:
Traunkreis Vet Clinic - Tierklinik Sattledt
Kirchdorfer Strasse 7
A-4642 Sattledt, Austria
Tel: +43 7244 8924
Email: office@tierklinik-sattledt.at
www.tierklinik-sattledt.at
He advises that he is able to provide travel and accommodation assistance, as well.
His exclusion criteria for surgery are:
• Severe pulmonary hypertension (more than 60 mm Hg)
• Clinical symptoms of syringomyelia
• Severe concomitant diseases (e.g. kidney failure, liver failure, malignant neoplasia)
RETURN TO TOP
• Dr. Poppy Bristow -- United Kingdom
NOTICE: Dick White Referrals (DWR) has announced that, effective December 6, 2023, Dr. Poppy Bristow has decided to cease performing open heart surgeries, and therefore DWR has halted its open heart surgery program for the foreseeable future.
 Dick
White Referrals (DWR), a specialist veterinary hospital in Cambridge, UK
offers open-heart mitral valve surgery to MVD-affected dogs, led by its
cardiac surgery head, Dr. Poppy Bristow (right), and its
cardiology head, Dr. Anne Kurosawa. DWR's contact information is:
Dick
White Referrals (DWR), a specialist veterinary hospital in Cambridge, UK
offers open-heart mitral valve surgery to MVD-affected dogs, led by its
cardiac surgery head, Dr. Poppy Bristow (right), and its
cardiology head, Dr. Anne Kurosawa. DWR's contact information is:
Dick White Referrals
Station Farm, London Road
Six Mile
Bottom
Cambridgeshire CB8 0UH
Tel: 01638 572012
Email:
reception@dwr.co.uk
www.dickwhitereferrals.com
RETURN TO TOP
• Drs. Karina Graham and Laurencie Brunel -- Australia
 Veterinary
Specialists of Sydney offers open-heart mitral valve surgery to
MVD-affected dogs, led by Dr. Karina Graham and Dr. Laurencie Brunel
(right). Contact information is:
Veterinary
Specialists of Sydney offers open-heart mitral valve surgery to
MVD-affected dogs, led by Dr. Karina Graham and Dr. Laurencie Brunel
(right). Contact information is:
Veterinary Specialists of Sydney
106 Parraweena Rd.
Miranda,
NSW 2228
Tel: (02) 8376 8767
Email:
info@vsos.com.au
www.vsos.com.au
RETURN TO TOP
• Dr. Sabine Bozon -- France
 In Guyancourt, France, Drs. Sabine Bozon
(right) and John-Hugues Bozon of Clinique Veterinaire Bozon,
who trained under Dr. Uechi, are providing open-heart surgeries for
mitral valve repairs.
In Guyancourt, France, Drs. Sabine Bozon
(right) and John-Hugues Bozon of Clinique Veterinaire Bozon,
who trained under Dr. Uechi, are providing open-heart surgeries for
mitral valve repairs.
Their contact information is:
Clinique Veterinaire HOPIA
14 & 15 BOULEVARD DES CHÊNES 78280
GUYANCOURT, France
Tel: 01 39 53 17 17
email: contact@hopia.fr
website: www.hopia.fr
RETURN TO TOP
For more information:
For more information about all of these open heart surgery programs, see the Mighty Hearts Project website. The box below lists locations where mitral valve surgeries have been performed, with details about their techniques, success rates, and costs.
RETURN TO TOP
Transcatheter edge-to-edge device (TEER) - V-clamp
-
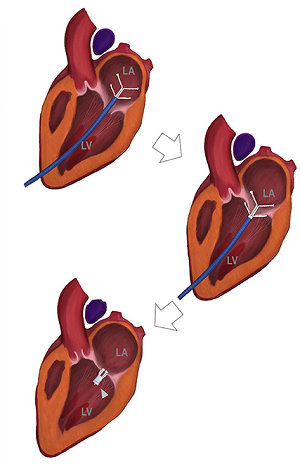 Colorado State
University (CSU)
Colorado State
University (CSU) - University of Minnesota
- University of Illinois
- Florida: Sawgrass Veterinary Cardiology
- VCA West Los Angeles Animal Hospital
- New York: Upstate Veterinary Specialties
- Bristol Vet Specialists -- UK
- Langford Vets -- UK
- Willows Veterinary Centre -- UK
- Ludwig-Maximilians University (LMU) -- Germany
- Veterinary Cardiologists - Australia
- Future locations
Minimally invasive surgeries are not "open heart" surgeries and do not require cardio-pulmonary by-pass equipment. They may be called "transcatheter interventions" with "catheter-delivered devices".
Transcatheter Edge-to-Edge Repair (TEER) of the mitral valve is a minimally-invasive surgical procedure intended to reduce or eliminate blood regurgitation back through the mitral valve leaflets. A clipping device is inserted in a thin tube (catheter) through a vein in the dog's leg, first into the left ventricle of the heart and then across the mitral valve into the left atrium under echocardiographic guidance. The clip is attached to the mitral valve to enable it to close more completely. (The diagram above shows, in the top image, the blue catheter inserted through the left ventricle [LV] and its white V-clamp device through the mitral valve into the left atrium [LA]. The second image shows the catheter pulled downwards with the V-clamp attached at both the top and bottom of the mitral valve leaflets. The third image shows the catheter removed and the V-clamp permanenently in place between the leaflets of the mitral valve.)
The TEER procedure is not suitable for every MVD patient. Suitability depends, in large part, upon the condition of the leaflets of the mitral valve. So, prior to undergoing TEER, the patient's valve must be examined thoroughly using transesophageal echocardiography (TEE). Since TEE requires that the dog be placed under anesthesia, the typical procedure is, one the day scheduled for TEER, the dog is placed under ansesthesia and undergoes the TEE echo examination. If the surgeon determines from that TEE exam that the valve leaflets are suitable for TEER, then he proceeds to perform TEER. If he determines that the dog's valve is not suitable, then the TEER is cancelled.
In a
December 2020 article, a team of Chinese veterinary surgeons
report the successful preliminary outcomes in the surgical implantation
of an edge-to-edge transcatheter device which they clamped on the mitral
valves of eight dogs to control regurgitation. (See diagram at
right.) All eight dogs had Stage
B1 mitral valve disease. The
 procedure required anesthesia but not
cardiopulmonary bypass. All reportedly survived without complications.
Echocardiography revealed a significant reduction in mitral
regurgitation severity in all dogs following the procedure based on both
a reduced mitral regurgitant maximum jet area and a reduced mitral
regurgitant maximum jet area to left atrial area. No major complications
had been reported in any of the dogs during the follow-up periods of a
range from 100 to 141 days. They concluded:
procedure required anesthesia but not
cardiopulmonary bypass. All reportedly survived without complications.
Echocardiography revealed a significant reduction in mitral
regurgitation severity in all dogs following the procedure based on both
a reduced mitral regurgitant maximum jet area and a reduced mitral
regurgitant maximum jet area to left atrial area. No major complications
had been reported in any of the dogs during the follow-up periods of a
range from 100 to 141 days. They concluded:
"[T]he dogs included in this study were limited to dogs with mitral regurgitation but no clinical signs and minimal, if any, chamber enlargement (stage B1 MMVD). The efficacy of this device still needs to be further tested on patients with varying stages of MMVD. ... In conclusion, the edge-to-edge valve closure using the ValveClamp mitral valve system is easy to perform and is highly effective at reducing the severity of mitral regurgitation. The system has good potential to be used in clinical practice on canine MMVD patients, although more data needs to be collected to prove its long-term safety and efficacy."
See also this March 2022 article, reporting successfully utilizing this Transcatheter Edge-to-Edge Repair (TEER) procedure on two healthy beagles.
 A
minimally-invasive procedure which is a form of the
TEER is the
V-clamp device. It was first
performed by a Milan, Italy cardiology team
led
by Dr. Claudio Bussadori (right). The device, which appears to be designed remarkably like the TEER device
and is inserted into the dog's mitral valve
A
minimally-invasive procedure which is a form of the
TEER is the
V-clamp device. It was first
performed by a Milan, Italy cardiology team
led
by Dr. Claudio Bussadori (right). The device, which appears to be designed remarkably like the TEER device
and is inserted into the dog's mitral valve
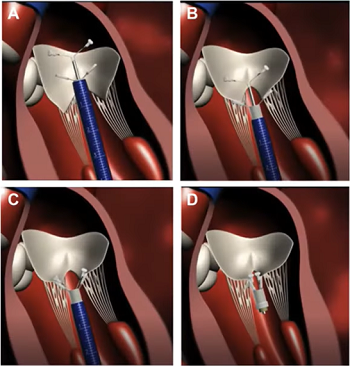 in the same manner as the
TEER, by means of a thin tube through a vein and into the left ventricle
of the heart. (See images at left.) The procedure is monitored
by transesophageal echocardiography and fluoroscopy.
in the same manner as the
TEER, by means of a thin tube through a vein and into the left ventricle
of the heart. (See images at left.) The procedure is monitored
by transesophageal echocardiography and fluoroscopy.
In a silent YouTube video, the entire procedure is viewable, from the pre-surgical echo examination of a 7-year old cavalier named Trevor to the dog's post-surgery echo and reported results. Following the surgery, Trevor's mitral regurgitation was reportedly "markedly reduced", with the his LA/Ao ratio reduced from 2.57 prior to surgery down to 1.3 afterwards. The dog was discharged from the clinic less than 24 hours after the surgery, and a week later, he reportedly was "doing well".
RETURN TO TOP
• Colorado State University (CSU) -- USA
 The
cardiology department at Colorado State University (CSU), led by Dr.
Christopher Orton (right) has been performing the
TEER procedure on
MVD-affected dogs since 2022. Eligible dogs must have servere mtral
regurgitation and be in advanced Stage B2 or Stage C of MVD. The cost of
this procedure, which includes pre-op evaluation and aftercare, is
between $6,000.00 and $8,000.00. For more information, contact Dr. Katie
Abbott-Johnson at
K.Abbott-Johnson@colostate.edu. See also
this webpage.
The
cardiology department at Colorado State University (CSU), led by Dr.
Christopher Orton (right) has been performing the
TEER procedure on
MVD-affected dogs since 2022. Eligible dogs must have servere mtral
regurgitation and be in advanced Stage B2 or Stage C of MVD. The cost of
this procedure, which includes pre-op evaluation and aftercare, is
between $6,000.00 and $8,000.00. For more information, contact Dr. Katie
Abbott-Johnson at
K.Abbott-Johnson@colostate.edu. See also
this webpage.
In a June 2023 abstract, the veterinary heart surgery team at CSU reported on the feasibility of their mitral V-clamp device. Over a two-year period 40 MVD-affected dogs (Stage B2: 12; Stage C: 26; Stage D: 2) were enrolled in the clinical study, an FDA-approved feasibility study. The Stage B2 dogs all had severe mitral regurgitation (MR). TEER was performed using transesophageal echocardiography and fluoroscopy. All dogs survived the surgical procedure. Thirty-eight (38) of the 40 dogs (95%) survived to discharge at the hospital. Most dogs were discharged within two days following surgery. The adverse event rate was three events (6.2%), which consisted of (a) a single-leaflet detachment, (b) one V-clamp unlocked, and (c) one clamp embolization to prevent abnormal bleeding. All three events were non-fatal and successfully treated with a second clamp. Nine months following hospital discharge, survival was 87.4% due to all causes and 91.1% cardiac-related. They concluded that: "Initial feasibility results support continued development of TEER as a low-risk and effective treatment for degenerative MR in dogs."
In a December 2024 article, the heart surgery team at CSU reported on the outcome of TEER surgeries on 50 dogs, 13 of which (26%) were cavalier King Charles spaniels. This was a feasibility study conducted from 2021 through April 2023. Overall, the procedural feasibility was 96% based on delivery of at least one device in 48 of the 50 dogs. Six other dogs were judged to be inappropriate for TEER, after being placed under anesthesia, based on unfavorable mitral valve functional anatomy or anterior-posterior dimension, and TEER was not attempted.
The V-Clamp device was not implanted in two dogs due to failure to capture the leaflets. Both dogs were discharged from the hospital. One of these dogs died of progressive heart failure 6 weeks after the surgery. The other dog died suddenly the day after hospital discharge. There were no deaths during the surgeries. Two dogs were euthanized before being discharged from the hospital, due to worsened mitral regurgitation after the surgery. Overall procedural success based on successful deployment of at least one clamp and discharge from the hospital was 92%. Nine dogs had two clamps delivered. One dog had three clamps delivered. The adverse device-related event rate was 6.3% based on three nonfatal incidents. All three events were attributed to operator error and inexperience. The authors concluded:
"In conclusion, initial feasibility results support continued development of TEER as a procedurally feasible, relatively low-risk, and low morbidity treatment for severe degenerative MR in dogs. Evidence of short-term efficacy is promising but will need verification with longer-term follow up. Operator experience, earlier intervention, and evolving understanding of favorable functional anatomy for edge-to-edge repair may result in the better reductions in MR severity in the future."
At an October 2025 presentation before the American College of Veterinary Surgeons (ACVS) summit, Dr. Orton updated the TEER statistics following a total of 134 cases, 35 patients (28%) being cavalier King Charles spaniels. See this chart (below) from his presentation for the details about those patients. He reported that 80% of the TEER dogs were alive a year after their procedure, and 78% were alive after two years. He said that following TEER, the average sizes of the left atrium (LA:Ao) and left ventricle (LVIDdN) are reduced, and that some of them return to the normal range. The regurgitant volume on average is reduced by more than 50%, and the regurgitation fraction is reduced from an average of over 60% to as low as 30% a year after the procedure.
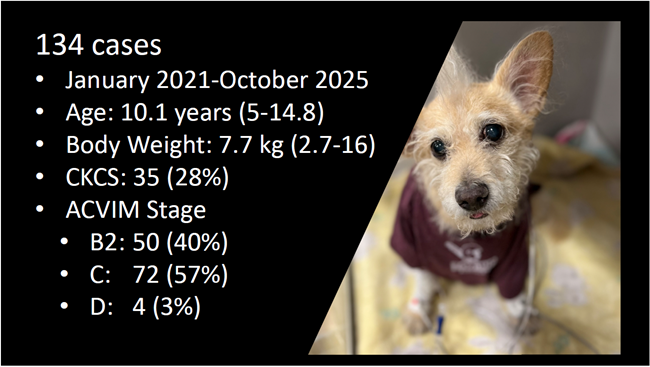
RETURN TO TOP
 •
University of Minnesota -- USA
•
University of Minnesota -- USA
In March 2023, the University of Minnesota's College of Veterinary Medicine announced it has performed three TEER procedures using the V-clamp device, two on cavaliers, and all successful. For more information, contact Dr. Allison Masters (right) at maste289@umn.edu. Dr. Masters has estimated the cost for performing this procedure at between $13,000.00 and $15,000.00.
RETURN TO TOP
• University of Illinois -- USA
In July 2023, the University of Illinois' College of Veterinary Medicine announced that it was beginning TEER treatment for dogs diagnosed with severe mitral regurgitation. To be eligible for this procedure, the patient must weigh between 4 to 15 kg and have evidence of ACVIM stage B2 or stage C. Dogs in late stage C/D or atrial fibrillation are considered poor candidates for this procedure. See this link for details. The most recent success/failure statistics (as of March 2025) are, out of 48 dogs, 36 survived the surgery and discharge and 12 have not.
For more information, contact the cardiology service at the Veterinary Teaching Hospital: vthcardiology@vetmed.illinois.edu
RETURN TO TOP
 •
Florida: Sawgrass
Veterinary Cardiology
•
Florida: Sawgrass
Veterinary Cardiology
Dr. Dewey Carpenter (right) at Sawgrass Veterinary Cardiology in Fort Lauderdale and Royal Palm Beach, Florida is performing TEER surgeries on MVD-affected dogs. See this link for details. Contact the clinic at 954-487-8357 or on its online contact form.
RETURN TO TOP
• VCA West Los Angeles Animal Hospital
The cardiology department of the VCA West Los Angeles Animal Hospital began offering TEER procedures for MVD-affected dogs in early 2023. The team is led by Dr. Justin Allen. See this link for details, and contact the team directly via email at wla.cardiology@vca.com
RETURN TO TOP
• New York: Upstate Veterinary Specialties
In December 2025, Dr. Cassidy D. Sedacca announced that his hospital, Upstate Veterinary Specialites is performing TEER proceduresusing the MitraClip device, with their first patient a cavalier King Charles spaniel named Emma. See this link for details. Contact them at 518-783-3198,
RETURN TO TOP
 •
Bristol Vet Specialists --
UK
•
Bristol Vet Specialists --
UK
In November 2025, Bristol Vet Speccialists, in Bristol, UK announced that its cardiologists, Eoin Kilkenny (right) and Kieran Borgeat, are offering TEER to MVD-affected dogs. Details are available on its website at this link.
RETURN TO TOP
• Langford Vets -- UK
 In May 2022, Langford Vets, Small Animal Referral Hospital, at the
University of Bristol in the UK announced that it is accredited to
perform the TEER procedure on small dogs (between 9 and 33 lbs.). They
explain that:
In May 2022, Langford Vets, Small Animal Referral Hospital, at the
University of Bristol in the UK announced that it is accredited to
perform the TEER procedure on small dogs (between 9 and 33 lbs.). They
explain that:
"All potential candidates will need to be fully assessed by our Cardiology team, who will perform a heart scan and have a consultation with owners prior to scheduling a surgery date. Referrals must come through a veterinary practice, and previous reports from your vets will not be able to be used for us to accurately assess suitability for the procedure. For more information and to enquire about a possible TEER case referral, please ask your vet to contact us by email, on cardio@langfordvets.co.uk."
Langford Vets' cardiology team is led by Dr. Melanie Hezzell (right).
RETURN TO TOP
• Willows Veterinary Centre -- UK
 In January 2023, Willows Veterinary Centre & Referral Service in the UK
announced that it is offering TEER- V-clamp minimally-invasive procedure
for MVD-affected dogs. Its team is led by Simon Swift (right), who said:
In January 2023, Willows Veterinary Centre & Referral Service in the UK
announced that it is offering TEER- V-clamp minimally-invasive procedure
for MVD-affected dogs. Its team is led by Simon Swift (right), who said:
"MMVD occurs when the mitral valve, which divides the left side of the heart into top and bottom chambers (atrium and ventricle respectively), starts leaking and creates a heart murmur. The leak can gradually become larger and, in the long term, often leads to dilation of the left-sided chambers. In time, the pressure in the left atrium starts rising and ultimately leads to development of fluid in the lungs, known as congestive heart failure or pulmonary oedema. Transcatheter edge-to-edge mitral valve repair (TEER) is already considered an alternative to surgical repair in human patients and there have been some very promising early clinical results in dogs. We are incredibly excited to be pioneering this TEER procedure at Willows in the treatment of dogs affected by advanced mitral valve disease. The procedure is performed under general anaesthesia through a small incision in the chest wall. Access within the beating heart is achieved by a needle puncture at the apex of the heart which is highlighted by continuous X-ray images and an ultrasound of the heart via a probe placed in the oesophagus. This allows correct positioning of a V-clamp across the mitral valve to reduce the amount of leakage. It's a procedure that delivers meaningful results and an improved quality of life and, because it's a minimally invasive process, patients are typically discharged from hospital within two days."
See this link for more information.
RETURN TO TOP
• Ludwig-Maximilians University (LMU) -- Germany
 In March 2024, in Munich, Germany, the Ludwig-Maximilians University
(LMU) small animal clinic announced that its cardiovascular team, led by
Dr. Gerhard Wess (right), is providing the Transcatheter Edge-to-Edge (TEER)
mitral clamp (V-Clamp) technique to MVD-affected dogs in Stage C and in
"advanced Stage B2", which they define as having these minimum
echocardiographic measurements:
In March 2024, in Munich, Germany, the Ludwig-Maximilians University
(LMU) small animal clinic announced that its cardiovascular team, led by
Dr. Gerhard Wess (right), is providing the Transcatheter Edge-to-Edge (TEER)
mitral clamp (V-Clamp) technique to MVD-affected dogs in Stage C and in
"advanced Stage B2", which they define as having these minimum
echocardiographic measurements:
• LAD (left atrial dimension) >1.9
• LA/Ao (left atrium dimension divided by aorta dimension) >1.9
• LVIDD-n (left ventricle dimension) >1.9
Contact the clinic at Kardio@med.vetmed.uni-muenchen.de
RETURN TO TOP
• Veterinary Cardiologists - Australia
In Australia, Veterinary Cardiologists Australia (VCA) is offering TEER - V-clamp mimimally-invasive surgeries to MVD-affected dogs. Their tesm is lead by Drs. Brad Gavaghan, Fiona Meyers, and Chris Lam. Small breed dogs (over 4 kg) with advanced valve disease (ACVIM Stage B2 or Stage C)* may be eligible for the procedure. See this link for details.
RETURN TO TOP
• Future locations
Other surgery facilities which are preparing to perform the V-clamp surgeries are:
• Tufts University Foster Hospital for Small Animal
RETURN TO TOP
• Transapical Beating Heart Mitral Valve Replacement
Transapical surgeries involve replacing the deteriorated mitral valve
with a bioprosthetic mitral valve while the
 heart
continues to function normally. The heart is not stopped or by-passed,
and it is not accessed through a vein. Instead, while the dog is under
anesthesia, an incision is made in the skin between the ribs, and the
heart is visible to the surgeons. This is called a thoracotomy.
heart
continues to function normally. The heart is not stopped or by-passed,
and it is not accessed through a vein. Instead, while the dog is under
anesthesia, an incision is made in the skin between the ribs, and the
heart is visible to the surgeons. This is called a thoracotomy.
A needle-like surgical probe, to which is attached the artifiical mitral valve (right), is inserted through the exterior wall at the apex of the heart, guided by 2D and 3D transesophageal echocardiography (TEE). Once the artificial valve is in place, the probe is withdrawn.
In February 2025, Sawgrass Veterinary Cardiology in Royal Palm Beach, Florida USA announced that Dr. Dewey Carpenter and his surgical team performed a successful transapical mitral valve replacement procedure on a dog, the first such surgery in the United States. They reported after 36 hours that the device "is showing excellent flow with zero regurgitation or perivalvular leakage." (See x-ray image below.)
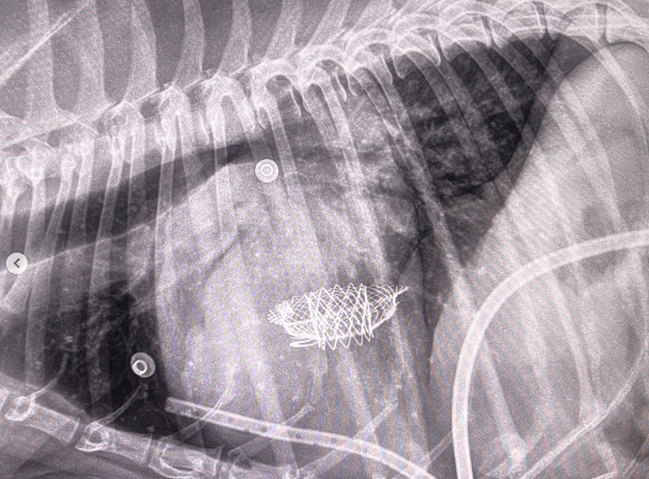
RETURN TO TOP
• Left Atrial Decompression (LAD)
Left atrial decompression (LAD) is a nimimally invasive surgical procedure in
which a small hole is created in the internal wall of the heart between
the left atrium and the right atrium.
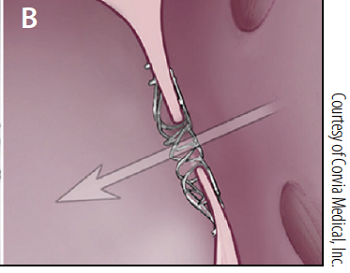 This
is called creating an acquired atrial septal defect, or
an atrial septostomy. The goal is to
reduce pressure in the enlarged by left atrium by allowing blood rom it
to flow directly into the right atrium. (See diagram at right.)
This is considered a last ditch effort to treat mainly dogs with
advanced congestive heart failure -- Stage D -- in which medications no
longer can compensate for the progression of the MVD.
This
is called creating an acquired atrial septal defect, or
an atrial septostomy. The goal is to
reduce pressure in the enlarged by left atrium by allowing blood rom it
to flow directly into the right atrium. (See diagram at right.)
This is considered a last ditch effort to treat mainly dogs with
advanced congestive heart failure -- Stage D -- in which medications no
longer can compensate for the progression of the MVD.
In a March 2021 article, a team of cardiologists at VCA West Los Angeles Animal Hospital reported the successful "immediate and substantial reduction in LAP" (left atrial pressure) in 17 dogs (including 3 [17.65%] cavaliers) with advanced CHF, by creating an intentional hole between the left atrium (LA) and the right atrium (RA), to allow blood in the LA to flow directly into the RA to relieve pressure in the LA. This procedure is called creating a "iatrogenic atrial septal defect", meaning an intentional one, and also is generally described as "left atrial decompression" (LAD). Atrial septal defects (ASDs) occur naturally in some dogs and usually require surgery to correct. An ASD is a hole directly between the left and right atriums, and naturally-formed ASDs interfere with the proper flow of blood through the heart.
Advanced MVD includes increased pressure in the LA, due to the backflow of blood through the mitral valve and causing the walls of the LA to enlarge. High left atrial pressure (LAP) is a precursor to CHF and persists following the onset of CHF. The 17 dogs described in this article all were in late Stage C or in Stage D of MVD, as they were not responding satisfactorily to cardiac medications. The LAD procedure involved inserting a needle through the chest wall and into a vein to the wall between the RA and the LA, and then inserting the needle into that wall to make a small hole -- usually 3 or more mm. -- to allow the more highly pressurized blood in the LA to flow directly into the RA and thereby relieve the overall pressure in the LA. An interatrial shunt device (iASD) is inserted between the two atrial chambers to maintain the opening and facilitate the flow of blood.
The investigators report that the "LAD resulted in a substantial and clinically relevant drop in LAP in all dogs that underwent the procedure." There were complications of the procedure, including deaths shortly afterwards in some cases, all of which were predictable due to the advanced MVD of the patients. Nevertheless, they concluded:
"In conclusion, LAD was a feasible procedure in patients of the present study with advanced MMVD and left-sided CHF and resulted in a substantial and clinically important reduction in LAP. In affected dogs, the LAD procedure may translate into improved patient quality of life and survival time, though this remains unknown. Complications were frequent but were manageable or preventable in most cases. Left atrial decompression can be performed in most catheterization laboratories with standard equipment, although a learning curve exists regarding location of the iASD and in minimizing complications. On the basis of findings of the present report, procedural success can be expected to improve with experience."
In a June 2023 abstract, the VCA West Los Angeles Animal Hospital cardiologists reported on the long term outcomes of 111 dogs surgically treated with LAD between October 2018 and September 2021. All of the dogs were diagnosed with severe mitral valve disease, including late Stage B2, Stage C, or Stage D. They report:
• 22 dogs (20%) had closure of the atrial septal defect surgically created by the team.
• 40 dogs (36%) developed right-sided congestive heart failure due to the LAD.
• 25 dogs (22.5%) had hospitalizations since their LAD procedures.
• Survival times following LAD were from 1 day to 1,282 days, with a median time of 379 days.
They concluded:
"Left atrial decompression was associated with a satisfactory survival time, given the severity of disease in the patient population. Right-sided congestive heart failure is common after the procedure, though onset is variable. Closure of the iatrogenic atrial septal defect is uncommon, and incidence can be minimized by ensuring transseptal puncture occurs within the fossa ovalis."
In a February 2025 article, clinicans described two case studies of LAD, one of which was a cavalier in heart failure due to MVD with severe mitral regurgitation (74%), the left atrium and left ventrile "markedly enlarged", pulmonary edema, and two ruptured chordae tendineae. Two months after the LAD procedure, the LA size and pressure had decreased and continued to improve for 4 more months. The dog remained free of CHF signs for 10 months and then died suddenly while asleep.
RETURN TO TOP
• Other Minimally Invasive Surgeries
- -- epicardial mitral annuloplasty device
- --- CoApt valve
- -- Tucker valve
- -- Harpoon TSD-5
- -- injections of alginate hydrogel
- -- vagus nerve stimulation
Minimally invasive surgeries are not "open heart" surgeries and do not require cardio-pulmonary by-pass equipment. They may be called "transcatheter interventions" with "catheter-delivered devices". Waht follows is a list of such minimally invasive surgeries, other than TEER - V-clamp.
--- epicardial mitral annuloplasty device
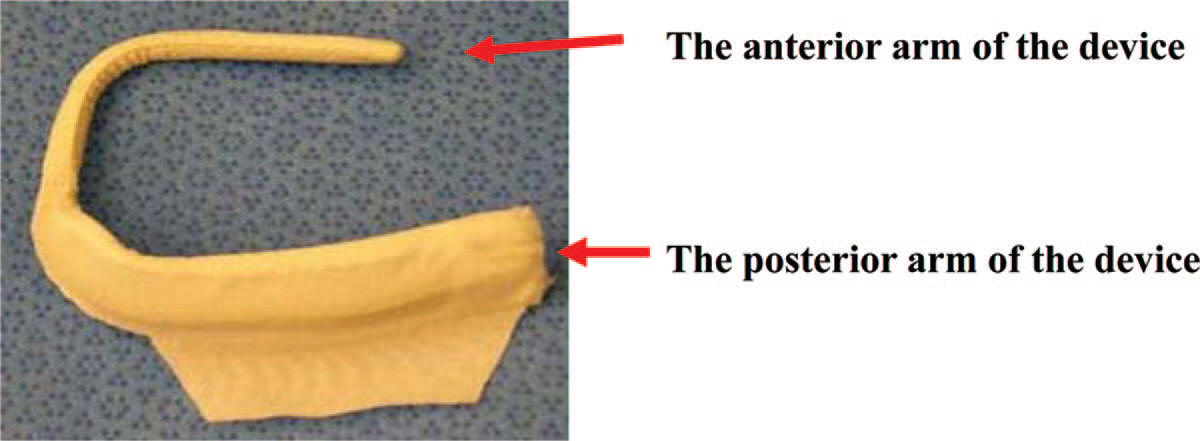 In
research, an implanted attachment to the mitral valve, called an "epicardial mitral annuloplasty device", has been
inserted on dogs' beating hearts in less than 30 seconds and without the need for cardiopulmonary
bypass surgery. In two published reports by researchers at the Cleveland
Clinic (January
2010 and
December 2010), the device (right) reduced the septal-lateral (S-L)
dimension of the mitral annulus (the ring that is attached to the mitral
valve leaflets) and eliminated the backflow of blood through the mitral
valve, without requiring
the use of a cardiopulmonary bypass procedure. See the diagram
below.
In
research, an implanted attachment to the mitral valve, called an "epicardial mitral annuloplasty device", has been
inserted on dogs' beating hearts in less than 30 seconds and without the need for cardiopulmonary
bypass surgery. In two published reports by researchers at the Cleveland
Clinic (January
2010 and
December 2010), the device (right) reduced the septal-lateral (S-L)
dimension of the mitral annulus (the ring that is attached to the mitral
valve leaflets) and eliminated the backflow of blood through the mitral
valve, without requiring
the use of a cardiopulmonary bypass procedure. See the diagram
below.
The device, called Mitral Touch, has been manufactured by MAQUET Cardiovascular LLC of San Jose, California. It consists of a titanium wire backbone, silicone bulking, and polyester fabric cover with a flap of 1 cm for securing it to the heart. The device is secured with titanium helical tacks driven through the device into the ventricular wall.
In a 2014 report on what appears to be the same device but now manufactured by Infiniti Medical and called Mitrex, researchers reported the results of a six month trial of the device implanted on the beating hearts of ten swine. Necropsy was performed at 180 days. The researchers found that:
"Coronary flow, ejection fraction, left ventricular wall motion and mitral valve function were normal post implantation and at term. ... Devices were well tolerated causing only minimal to mild fibrosis and chronic inflammation. No significant changes were observed in the myocardium except for muscle fiber atrophy near the tip of the anterior arm. There appeared to be ample tissue over the tip and no danger of perforation in all but one subject. No meaningful changes were noted in cardiac shape, ventricular wall thickness, chamber size, heart valves, and blood vessels. The myocardial compression necessary to perform epicardial annuloplasty was well tolerated. The Mitrex device was safe and biocompatible."
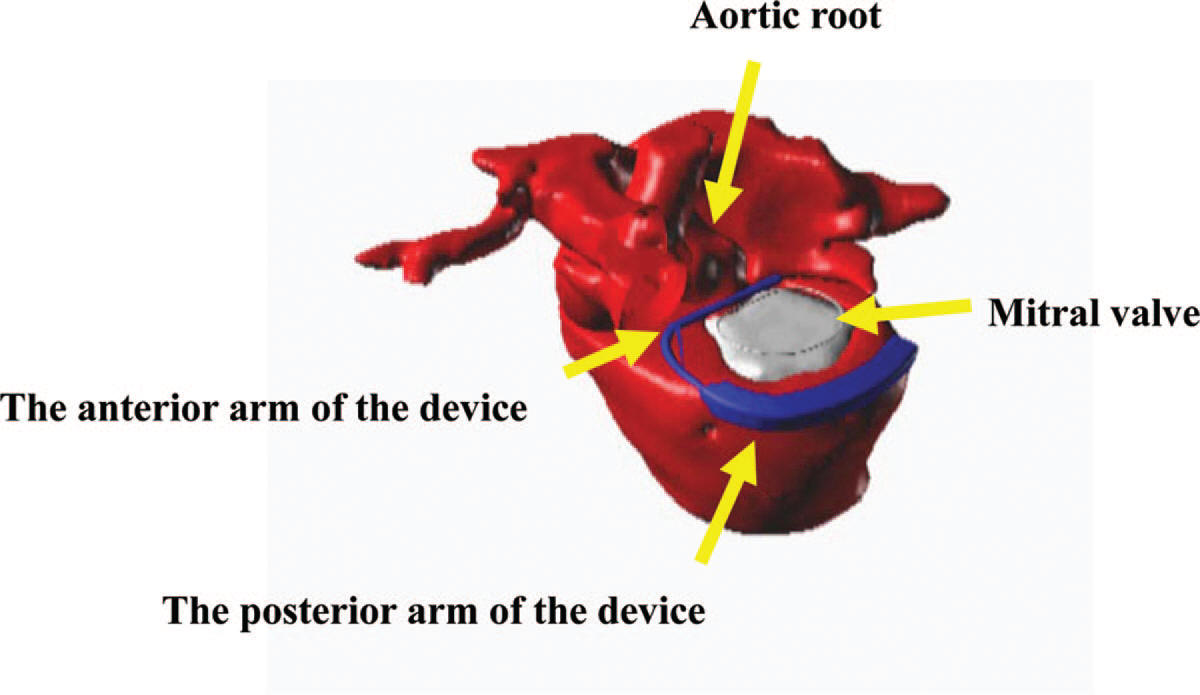
This same report also was presented to the June 2015 ACVIM Forum. The device currently is being tested by veterinary cardiac surgeons at clinics in California and Florida on MVD-affected dogs in congestive heart failure.
RETURN TO TOP
 ---
CoApt
valve
---
CoApt
valve
Ultravet Medical Devices, led by board certified veterinary cardiologist Dr. George Kramer, is researching the viabilitity of a device, called the CoApt valve (right), which is designed to be inserted through a vein into the left ventricle chamber. A YouTube video of an animated diagram of how the CoApt valve is to be inserted and to operate is here.
RETURN TO TOP
 ---
Tucker valve
---
Tucker valve
Ultravet Medical Devices also has been researching the viabilitity of a device called the Tucker valve (named after his dog), which is inserted through either the mitral or the tricuspid valve in order to block regurgitation of blood through the valve back into the atrium. An animated diagram of how the Tucker valve is to be inserted and to operate is here. See this November 2015 article about the Tucker valve. (The animated out-take at the right shows the Tucker valve through the tricuspid valve.)
RETURN TO TOP
--- Harpoon TSD-5
Dr. Michele Borgarelli at Virginia Tech vet school is testing the feasibility of minimally invasive repair of the mitral valve using the Harpoon TSD-5 device developed by Harpoon Medical Inc. of Baltimore, Maryland. The device is designed to anchor artificial chords to the leaflets of the valve, to replace the vavle's natural chordae tendineae. The replacement cords are made of expanded polytetrafluoroethylene (ePTFE).
In a May 2017 article, Borgarelli reported the successful implantation of ePTFE replacement mitral valve chords in four of six healthy Beagles, using minimally-invasive surgical techniques in beating hearts (without cardio-pulmonary bypass or cardiac arrest). The investigators used a biomedical device called the Harpoon Medical TSD-5. The body cavity was opened to reveal the beating heart. Then a "valved introducer" was inserted into the left ventricle, and the thin metal shaft of the Harpoon TSD-5 was inserted through the introducer, leading to the mitral valve. When the shaft was in proper position, the trigger of the Harpoon TSD-5 (see image below) was pressed, and its needle pierced the mitral valve leaflet, forming a bulky knot of the ePTFE chord through the leaflet. The other end of the chord ultimately is extended and attached to the heart muscle through the intial insertion location. This video shows how the procedure is designed to operate. The study authors concluded:
"This pilot study has demonstrated feasibility of using the Harpoon TSD-5 device to place and anchor ePTFE artificial chords to the MV of small dogs and that endothelialization of the synthetic cord and knots can start within 30 days."
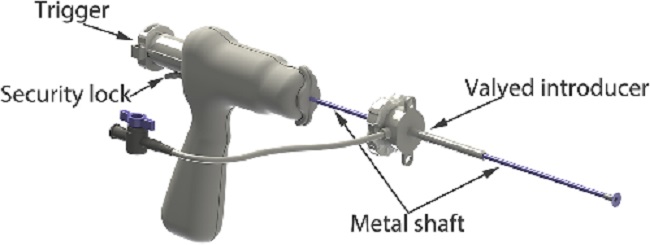
As of June 2017, Dr.Borgarelli is seeking candidate dogs for a study to assess the efficacy and long-term effects of using the Harpoon Medical TSD-5 device. Dogs in Stage C, heart failure with significant enlargement, and weighing over 11 pounds. Details of the study are available here.
RETURN TO TOP
--- injections of alginate hydrogel
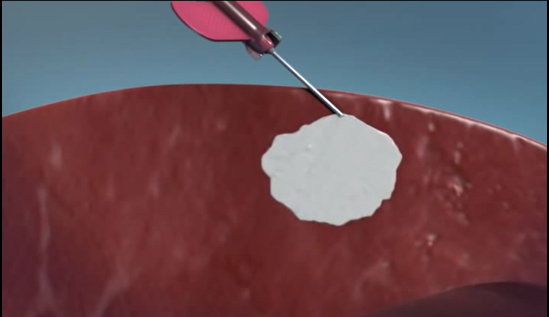 In
a
June 2013 study by a team of US cardiologists, they found that
injecting alginate hydrogel directly into the left
ventricle (LV) of the hearts of 7 dogs in advanced heart failure,
increased the thickness of the LV wall, and LV structure and function
improved. Alginate is a naturally derived polysaccharide that is used in
drug delivery and as cell encapsulation material. (See a diagram of
the injection at right.) The brand name
Algisyl-LVR is manufactured by LoneStar Heart, Inc. of Laguna
Hills, California.
In
a
June 2013 study by a team of US cardiologists, they found that
injecting alginate hydrogel directly into the left
ventricle (LV) of the hearts of 7 dogs in advanced heart failure,
increased the thickness of the LV wall, and LV structure and function
improved. Alginate is a naturally derived polysaccharide that is used in
drug delivery and as cell encapsulation material. (See a diagram of
the injection at right.) The brand name
Algisyl-LVR is manufactured by LoneStar Heart, Inc. of Laguna
Hills, California.
See also these 2009 and August 2010 articles by the same research team.
RETURN TO TOP
--- vagus nerve stimulation
 Vagus,
the tenth cranial nerve, controls a broad range of organs in the head,
neck, and thorax,
including the cardiac, pulmonary, esophagus, and gastrointestinal tract
regions. It travels from the head along the carotid artery. The vagus nerve is responsible for the heart rate by secreting
a substance called acetylcholine. Vagus nerve stimulation (VNS) therapy,
an electrophysiology technique in which pulses of electrical activity
are supplied to the vagal nerve,
using a generator device (e.g., at right) implanted in the chest
with its wires wrapped around the nerve, to stimulate
branches of the vagus to control such things as seizures in epilepsy
patients and clinical depression.
Vagus,
the tenth cranial nerve, controls a broad range of organs in the head,
neck, and thorax,
including the cardiac, pulmonary, esophagus, and gastrointestinal tract
regions. It travels from the head along the carotid artery. The vagus nerve is responsible for the heart rate by secreting
a substance called acetylcholine. Vagus nerve stimulation (VNS) therapy,
an electrophysiology technique in which pulses of electrical activity
are supplied to the vagal nerve,
using a generator device (e.g., at right) implanted in the chest
with its wires wrapped around the nerve, to stimulate
branches of the vagus to control such things as seizures in epilepsy
patients and clinical depression.
VNS also has been used experimentally to improve left ventricular dysfunction and slow the progression of heart failure in dogs. In a September 2009 article, a research team at the Cleveland Clinic performed VNS in 8 of 15 dogs and reported that, "chronic VNS improves cardiac autonomic control and significantly attenuates HF [heart failure] development in the canine high-rate ventricular pacing model. The therapeutic benefit of VNS is associated with pronounced anti-inflammatory effects. VNS is a novel and potentially useful therapy for treating HF." In a January 2014 study by the same team, they reported that: "VNS improved LA [left atrial] function and volumes and suppressed LA fibrosis in the canine high-rate ventricular pacing model. VNS is a novel and potentially useful therapy for improving LA function during HF."
RETURN TO TOP
Anesthesia
 In general,
MVD-affected dogs affected may have an increased risk to
anesthesia (anaesthesia) and sedatives. Pre-anesthetic evaluation, premedication,
induction, maintenance of anesthesia (anaesthesia), and monitoring of
anesthetized dogs and possible complications need to be taken into
account.
In general,
MVD-affected dogs affected may have an increased risk to
anesthesia (anaesthesia) and sedatives. Pre-anesthetic evaluation, premedication,
induction, maintenance of anesthesia (anaesthesia), and monitoring of
anesthetized dogs and possible complications need to be taken into
account.
The main aims for managing anesthesia in MVD-affected dogs are:
• Maintenance of a high-normal heart rate;
• Maintenance of adequate cardiac output; and
• Providing mild vasodilation.
The dog's heart rate should be close to the normal rate obtained during the pre-anesthetic examination, in order to maintain cardiac output. Cardiac output needs to be maintained to insure adequate delivery of oxygen to the body's tissues. Mild vasodilation (widening of the blood vessels, particularly the arteries) is desirable to optimize cardiac output.
The pre-anesthetic physical examination of the MVD-affected dog should be thorough. Screening including echocardiography (ideally by a cardiologist) and bloodwork (hematology, biochemistry, electrolytes). Pre-anesthetic evaluation also should include identifying any risk factors for worsening MVD, including coughing, rapid respiration, exercise intolerance, syncope, and/or pre-syncope (collapse). -- essentially any symptoms of an MVD-affected dog in current Stage C or D.
For more information, see our webpage on anesthesia and sedatives.
RETURN TO TOP
Tricuspid Valve Disease
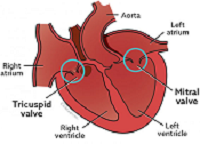 The tricuspid valve is between the right atrium and right ventricle, as
the mitral valve is between the left atrium and left ventricle. The
tricuspid valve controls the flow of blood from
the right atrium into the right ventricle. In some cases of MVD, as they
progress towards CHF, with pulmonary
hypertension, the tricuspid valve also begins to leak, causing blood
to backflow from the right ventricle into the right atrium.
The tricuspid valve is between the right atrium and right ventricle, as
the mitral valve is between the left atrium and left ventricle. The
tricuspid valve controls the flow of blood from
the right atrium into the right ventricle. In some cases of MVD, as they
progress towards CHF, with pulmonary
hypertension, the tricuspid valve also begins to leak, causing blood
to backflow from the right ventricle into the right atrium.
The tricuspid valve can experience the same deterioration as does the mitral valve, and this commonly is called tricuspid valve disease (TVD). As with the mitral valve, murmurs can be detected via the stethoscope when blood backflows from the right ventricle to the right atrium. These murmurs are graded the same way mitral valve murmurs are.
Prior to TVD's version of heart failure (right-sided CHF), TVD is treated in the same
fashion as is MVD. When TVD reaches the
 point of heart failure, fluid will build up in
the dog's abdomen and organs below the heart, such as the liver, spleen,
stomach, and gastrointestinal tract, causing enlargement of those
organs. The build up of fluid is called ascites or
abdominal effusion. At this point, TVD also is treated
the same as is Stage C of MVD, paticularly
with diuretics.
point of heart failure, fluid will build up in
the dog's abdomen and organs below the heart, such as the liver, spleen,
stomach, and gastrointestinal tract, causing enlargement of those
organs. The build up of fluid is called ascites or
abdominal effusion. At this point, TVD also is treated
the same as is Stage C of MVD, paticularly
with diuretics.
Ascites
gives the dog a heavy, pot-bellied
appearance. See photo at
right of cavalier, Kopi, with ascites (courtesy
Mount Pleasant Vet Group).
 If
the fluid retention is affecting the dog's ability to breathe, and
diuretics are not sufficient to ease the breathing issue, then these
retained fluids must be removed periodically by aspiration with a hypodermic needle, a process called
abdominocentesis. The needle is inserted into the
abdominal wall to drain excess fluid so that the dog will be more
comfortable and breathe more easily. (See photo of abdominocentesis in
process at left, courtesy
Mount Pleasant Vet Group.)
If
the fluid retention is affecting the dog's ability to breathe, and
diuretics are not sufficient to ease the breathing issue, then these
retained fluids must be removed periodically by aspiration with a hypodermic needle, a process called
abdominocentesis. The needle is inserted into the
abdominal wall to drain excess fluid so that the dog will be more
comfortable and breathe more easily. (See photo of abdominocentesis in
process at left, courtesy
Mount Pleasant Vet Group.)
 An x-ray prior to the
procedure, or use of ultrasound during the procedure, to find the fluid
pocket, is recommended, to avoid cutting into an organ in an unexpected
location. Alternatively, a larger surgical incision may be necessary,
called a laboratomy, to drain the fluid. See at right
the comparison photos of a dog with ascites before and after draining of
the fluid.
An x-ray prior to the
procedure, or use of ultrasound during the procedure, to find the fluid
pocket, is recommended, to avoid cutting into an organ in an unexpected
location. Alternatively, a larger surgical incision may be necessary,
called a laboratomy, to drain the fluid. See at right
the comparison photos of a dog with ascites before and after draining of
the fluid.
Many cardiologists prefer increasing diuretic doses rather than frequent draining of fluids. However, if the fluids reach high pressures, they can cause respiratory distress, which would require draining. Many ascites patients also eventually will suffer severe weight loss (cardiac cachexia).
The dog with right-sided heart failure also likely will have jugular puslsation when their pulses are examined.
RETURN TO TOP
Breeders' Responsibilities
 Early-onset mitral valve disease has been found to be
"highly heritable" in the cavalier King Charles spaniel breed, and
"selection against the disease should be successful.", according to an
April 2011
research report.
Early-onset mitral valve disease has been found to be
"highly heritable" in the cavalier King Charles spaniel breed, and
"selection against the disease should be successful.", according to an
April 2011
research report.
Due to the pervasiveness of MVD in the breed worldwide, cavalier King Charles spaniels under the age of five years should not be bred (with one limited exception -- see MVD Breeding Protocol). Also, no cavalier should be bred after age five years if it developed an MVD murmur before the age of five years. Any littermates of breeding stock having early-onset MVD (mitral valve murmurs before age 5 years) should be taken into very serious consideration. All CKCS breeding stock should be examined by board certified veterinary cardiologists at least annually and cleared by the veterinary specialists for MVD, the closer the examination to the breeding the better. It is recommended that all cavaliers, breeding stock or not, be examined annually by board certified veterinary cardiologists after age one year. See the current list of health clinics for upcoming cardiologist examinations.
RETURN TO TOP
 What You Can Do
What You Can Do
- Annual heart checks
- When to get that first chest x-ray
- Count the breaths per minute
- Avoid vaccines
- Portable emergency oxygen kit
Annual heart checks
Since mitral valve disease (MVD) is so common in cavalier King Charles spaniels and is their leading cause of death, their hearts should be examined at least annually even before any indications of MVD arise. So, have their regular veterinarians listen to their hearts with a stethoscope for possible murmurs at each examination. See our section on Heart Murmur Grades for more information.
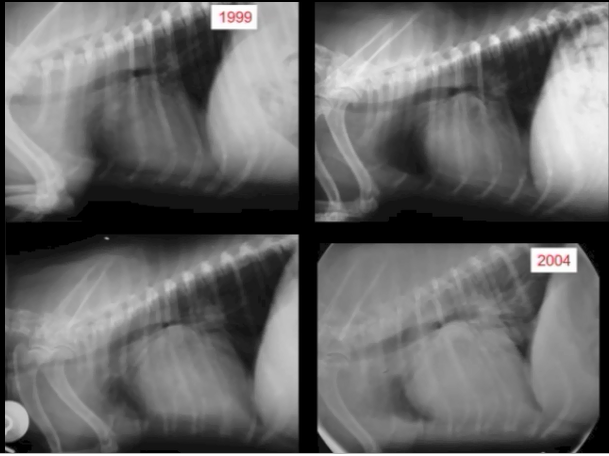 When
to get that first chest x-ray
When
to get that first chest x-ray
The first time the veterinarian detects a murmur over the mitral valve, the owner should have the cavalier's chest x-rayed. This first set of x-rays will serve as the "baseline" of the heart at its normal size, for comparing later x-rays to determine if the heart has begun to enlarge. A significantly enlarged heart (Stage B2) determines when to start medicating MVD with pimobendan. So, the ability to accurately compare old and new chest x-rays is very important.
Count the breaths per minute
A method which cavalier owners can use to determine if and when their MVD-affected dog reaches Stage C, the stage of heart failure, is to count the dog's breaths per minute while sleeping. Researchers have found that healthy adult dogs generally have an average sleeping respiratory rate of less than 30 breaths per minute and rarely exceed that rate at any time. Some cardiologists recommend that their patient's owners periodically count their dog's sleeping respiratory rate, and when the average rate starts to creep up to the 30s, to make an appointment for the dog to be re-examined by the cardiologist to see if the dog is approaching or has reached the stage of heart failure. Note that, ideally, the dog must be asleep and not appear to be dreaming, for an accurate respiratory rate. See our section on Respiratory Rates for more information.

Avoid vaccines
 Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.) A dog
diagnosed with MVD, especially in Stage B2 or above, is not a healthy dog.
Vaccine manufacturers provide warnings on their
products' data sheets and labels, advising that only healthy dogs should be
vaccinated. (Click on the thumb-nail image at
right to view a typical warning on a rabies vaccine data sheet.) A dog
diagnosed with MVD, especially in Stage B2 or above, is not a healthy dog.
Some veterinarians recommend that dogs with advanced mitral valve disease -- Stage B2 or C -- not be vaccinated with the usual serums, including rabies, because of possible adverse reactions which might accelerate damage to the dogs' hearts. In such cases, the veterinarians will write letters to the county licensing authorities which require periodic vaccinations, and in many instances, the counties will accept the veterinarians' letters and excuse the dogs from having to be vaccinated.
For example, Dr. Larry Glickman, veterinary immunologist at Purdue University's veterinary school, wrote regarding cavaliers:
"Our ongoing studies of dogs show that following routine vaccination, there is a significant rise in the level of antibodies dogs produce against their own tissues. Some of these antibodies have been shown to target the thyroid gland, connective tissue such as that found in the valves of the heart, red blood cells, DNA, etc."
 Portable
emergency oxygen kit
Portable
emergency oxygen kit
In cases of emergency trips to the veterinarian when a Stage D dog experiences rapid respirations, a portable oxygen kit is available with an oxygen tank capable of lasting about 20 minutes. Paw Print Oxygen offers these kits without requiring a veterinarian's prescription.


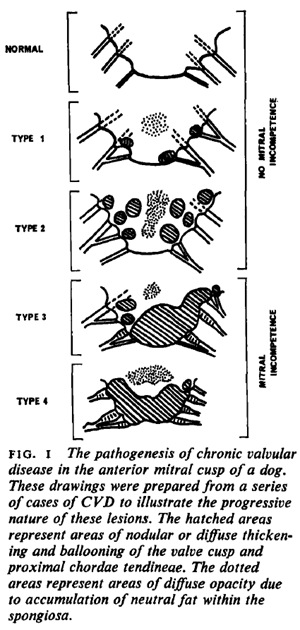 Deformity
Deformity
CONNECT WITH US